Abstract
Aim
SOX18 is a potential oncogene in osteosarcoma via controlling osteosarcoma cell proliferation and metastasis. Interleukin-6 (IL-6), a major activator of Janus kinase 2 (JAK2)/signal transducers and activators of transcription 3 (STAT3) signaling, plays an important role in the growth of carcinoma cells. The present study aims to investigate the correlation between IL-6 and SOX18 in osteosarcoma.
Materials and methods
Protein expression and mRNA expression were determined by Western blot and real-time polymerase chain reaction (PCR) analysis, respectively. Cell proliferation and apoptosis were identified by Cell Counting Kit-8 assay and flow cytometry analysis, respectively.
Results
We found that SOX18, IL-6 and p-STAT3 were elevated in osteosarcoma compared with bone cyst tissues. A positive correlation between the mRNA levels of IL-6 and SOX18 was observed in osteosarcoma tissues. IL-6 stimulation dose dependently induced the mRNA and protein levels of SOX18 in U-2OS and MG63 cells. Furthermore, IL-6 significantly rescued the inhibitory and induction effects of SOX18 knockdown on osteosarcoma cell proliferation and apoptosis, respectively. The changes in cell proliferation (PCNA) and apoptosis-related proteins (Bcl-2, Bax and Cleaved-Caspase 3) were in line with the results of cell proliferation and apoptosis assays.
Conclusion
Our data suggest that IL-6 is a possible upstream regulator for SOX18 in osteosarcoma.
Introduction
Osteosarcoma is the most common malignant bone-forming tumor.Citation1 Unlike other tumors, osteosarcoma usually occurs during the second and third decades of life. Despite the advances in surgery and chemotherapy for the past 2 decades, the survival time has hardly been improved.Citation2 As such, understanding the molecular mechanisms involved in the development of osteosarcoma is crucial for developing new molecular targets of diagnosis and effective therapy.
SOX18 belongs to sex-determining region on the Y chromosome-related high-mobility group box (SOX) gene family.Citation3,Citation4 SOX genes are highly conserved across species. SOX genes encode a group of transcription factors participating in developmental processes.Citation5 Recently, several studies have revealed the role of SOX18 in carcinogenesis. SOX18 expression is elevated in gastric cancerCitation6 and pancreatic ductal adenocarcinoma (PDAC).Citation7 Overexpression of SOX18 indicates poor prognosis for a wide spectrum of human malignancies, such as gastric cancer,Citation6 invasive ductal breast carcinoma,Citation8 ovarian cancerCitation9 and non-small cell lung cancer.Citation10 In our previous paper, we have showed that SOX18 is upregulated in osteosarcoma and served as a potential oncogene in osteosarcoma via regulating osteosarcoma cell proliferation and metastasis.Citation11 However, little is known about the upstream regulators of SOX18.
Interleukin-6 (IL-6), a proinflammatory cytokine, is a major activator of Janus kinase 2 (JAK2)/signal transducers and activators of transcription 3 (STAT3) signaling.Citation12 STAT3 is a vital transcription factor that participates in the regulation of cell proliferationCitation13 and cell apoptosis.Citation14 It has been identified as an oncogene in a variety of tumor types.Citation15 The total and phosphorylated forms of STAT3 are elevated in osteosarcoma cell lines and tissues. The increased level of phosphorylated STAT3 is associated with the poor prognosis in patients with osteosarcoma.Citation16 Expression of other SOX genes, such as SOX2,Citation17,Citation18 is mediated by STAT3. However, whether IL-6/STAT3 regulates SOX18 expression in osteosarcoma is unknown.
In the present study, upregulation of IL-6 and SOX18 was observed in human osteosarcoma. The mRNA level of SOX18 had a positive correlation with that of IL-6 in osteosarcoma tissues. Cell proliferation and apoptosis assays suggested that the IL-6 was a possible upstream regulator for SOX18.
Materials and methods
Tissue samples
From 2011 to 2014, 50 patients with osteosarcoma and 20 patients with bone cyst admitted to Shanghai Tenth People’s Hospital were enrolled in this study. This study was approved by the ethics committee of Shanghai Tenth People’s Hospital, Tongji University. Written informed consent was obtained from every participant and complied with the guidelines of the ethics committee. Osteosarcoma and bone cyst samples were collected and immediately frozen in liquid nitrogen and stored at −80°C until use.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR) assay
The mRNA levels of SOX18 were evaluated by real-time quantitative RT-PCR assay as previously described.Citation11 In brief, total RNA was extracted from tissue samples or cell lines with TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA, USA) and then reverse transcribed into complementary DNA (cDNA) by using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) per the manufacturer’s instructions. Real-time PCR was carried out with SYBR Green PCR kit (Thermo Fisher Scientific) by ABI 7300 instrument (Thermo Fisher Scientific). SOX18 expression levels were determined by normalization to internal control, GAPDH.
Western blot
Tissue samples (~200 mg) were ground into fine powder in liquid nitrogen using a mortar. Frozen tissue powder and cell lines were lysed in radio immunoprecipitation assay buffer (Beyotime, Shanghai, China) containing proteinase inhibitor cocktail (Sigma-Aldrich Co., St Louis, MO, USA), placed on ice for 30 min and then centrifuged at 13,000 rpm for 20 min at 4°C. The collected supernatant was quantitated by BCA Protein Assay Kit (Thermo Fisher Scientific). Equal amounts of protein (30 μg) was separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred electrophoretically to a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA). To block nonspecific binding, the membrane was incubated with 5% skim milk at room temperature for 30 min. Following incubation with primary antibodies at 4°C overnight, the membrane was incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (Beyotime). Protein expression was then analyzed using enhanced chemiluminescence (ECL; EMD Millipore) and ImageJ software (National Institutes of Health, Bethesda, MA, USA). GAPDH was detected as loading control. Sources of primary antibodies were as follows: 1) SOX18, p-STAT3, PCNA and Cleaved-Caspase 3 (Abcam, Cambridge, MA, USA); 2) STAT3 and GAPDH (Cell Signaling Technology, Danvers, MA, USA) and 3) Bcl-2 and Bax (Santa Cruz Biotechnology Inc., Dallas, TX, USA).
Cell culture
U-2OS, MG63 and HEK293T cells were obtained from American Type Culture Collection (Manassas, VA, USA). U-2OS cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific), while MG63 and HEK293T cells were grown in the Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific). Both media were supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 1% antibiotic (penicillin/streptomycin). All cell lines were maintained at 37°C in a 5% CO2 atmosphere.
Silencing of SOX18 by short hairpin RNA (shRNA)
shRNA targeting SOX18 (RNA interference [RNAi], TACCACGTGGCACTGGCCATT) and a nonspecific scramble shRNA sequence (NC, TTCTCCGAACGTGTCACGTTT) was cloned into PLKO.1 (Addgene, Cambridge, MA, USA). Recombinant lentiviruses were produced by transfecting the lentiviral construct and package plasmids into HEK293T cells as describedCitation11 and collected to transduce U-2OS and MG63 cells.
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was evaluated by using CCK-8 Assay Kit (Beyotime) following the manufacturer’s protocol. U-2OS and MG63 cells seeded in 96-well plates (1,000–1,500 cells/well) were transduced with shRNAs and treated with or without 50 ng/mL IL-6 (Sigma-Aldrich Co.). After incubating for 0, 24, 48 and 72 h, CCK-8 solution was added to each well and then incubated for 1 h. Optical density (OD) values at the wavelength of 450 nm were measured with a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA). All experiments were conducted in triplicate and repeated at least three times.
Cell apoptosis assay
Cell apoptosis rate was assessed by Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining kit (BD Biosciences, Franklin Lakes, NJ, USA). U-2OS and MG63 cells were transduced with shRNAs and treated with or without 50 ng/mL IL-6 (Sigma-Aldrich Co.) for 24 h. At the end of culture period, both adherent and floating cells were harvested and then labeled with Annexin V-FITC and PI. Cell apoptosis was then analyzed using a FACScan instrument (BD Biosciences).
Statistical analysis
All data are presented as mean ± SD. Statistical significance was determined by one-way analysis of variance (ANOVA). A P-value <0.05 was considered as statistically significant.
Results
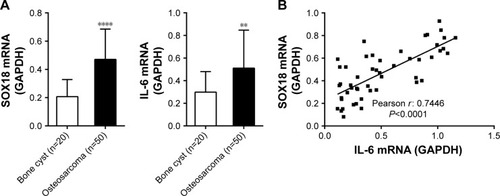
SOX18 expression was positively correlated with IL-6 expression in osteosarcoma tissues
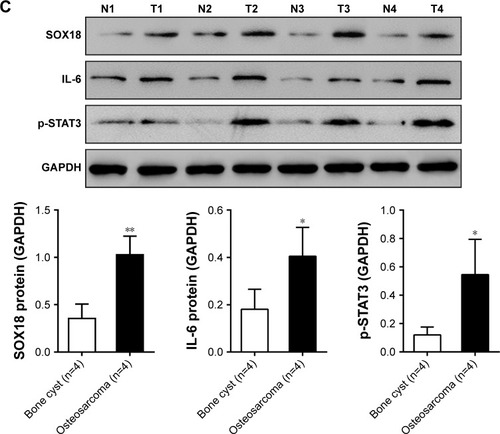
The mRNA expression of SOX18 and IL-6 was determined in osteosarcoma (n=50) and control bone cyst tissues (n=20). Comparing with bone cyst tissues, SOX18 and IL-6 mRNA levels increased by 127.5% and 71.4%, respectively (). Pearson correlation analysis was then performed to assess whether any relationship existed between the mRNA levels of SOX18 and IL-6 in osteosarcoma tissues. As shown in , the SOX18 mRNA level was positively correlated with the IL-6 level (r=0.7446, P<0.001). The protein levels of SOX18, IL-6 and p-STAT3 were also evaluated in osteosarcoma and bone cyst tissues. Osteosarcoma tissues had higher protein levels of SOX18, IL-6 and p-STAT3 as compared to bone cyst tissues (). These data indicated an association between IL-6/STAT3 and SOX18 during osteosarcoma progression.
Figure 1 SOX18 mRNA expression was correlated with IL-6 mRNA expression in osteosarcoma tissues.
Abbreviation: IL-6, interleukin-6.
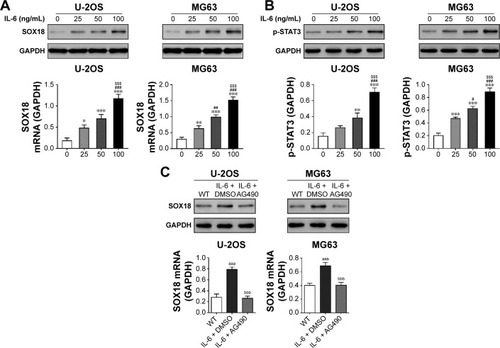
IL-6/STAT3 induced SOX18 expression
To determine whether IL-6 affected SOX18 expression in osteosarcoma, we treated U-2OS and MG63 cells with recombinant IL-6 and found that IL-6 concentration dependently enhanced the mRNA and protein levels of SOX18 (). These data suggested that IL-6 may induce SOX18 expression at the transcriptional level. Moreover, IL-6 treatment increased the levels of p-STAT3 in a concentration-dependent manner (). Additional AG490 (a STAT3 inhibitor) exposure significantly attenuated IL-6-induced SOX18 expression (). These results suggested that IL-6/STAT3 induced SOX18 transcription in osteosarcoma cells.
Figure 2 IL-6 increased SOX18 expression.
Abbreviations: PCR, polymerase chain reaction; DMSO, dimethyl sulfoxide; WT, without any treatment; IL-6, interleukin-6.
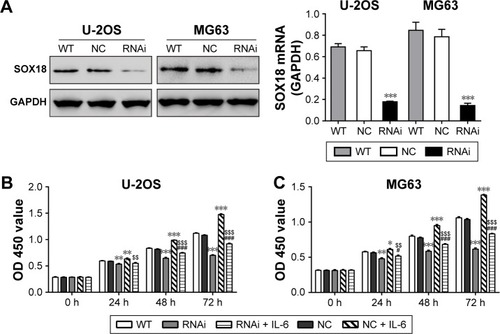
IL-6 promoted osteosarcoma cell proliferation via SOX18
Several investigators have reported that IL-6 can modulate the proliferation of carcinoma cells.Citation19–Citation21 To investigate whether IL-6 exerted functions through SOX18, we knocked down SOX18 expression in two osteosarcoma cell lines, U-2OS and MG63, by RNAi as previously reported ().Citation11 As displayed in , IL-6 exposure remarkably induced cell proliferation and such effect was notably attenuated by SOX18 knockdown.
Figure 3 IL-6 promoted osteosarcoma cell growth via SOX18.
Abbreviations: RNAi, RNA interference; CCK-8, Cell Counting Kit-8; WT, without any treatment; OD, optical density; IL-6, interleukin-6.
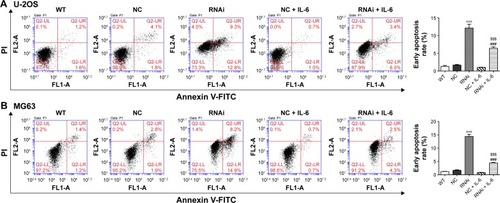
IL-6 exposure attenuated the induction effects of SOX18 knockdown on osteosarcoma cell apoptosis
Cell apoptosis was evaluated by Annexin V-FITC/PI staining assay. As shown in , knockdown of SOX18 in U-2OS or MG63 cells significantly induced cell apoptosis in comparison with corresponding scramble shRNA. The presence of IL-6 significantly reduced SOX18 knockdown-induced cell apoptosis.
Figure 4 IL-6 exposure attenuated the induction effects of SOX18 knockdown on osteosarcoma cell apoptosis.
Abbreviations: WT, without any treatment; RNAi, RNA interference; FITC, fluorescein isothiocyanate; PI, propidium iodide; IL-6, interleukin-6.
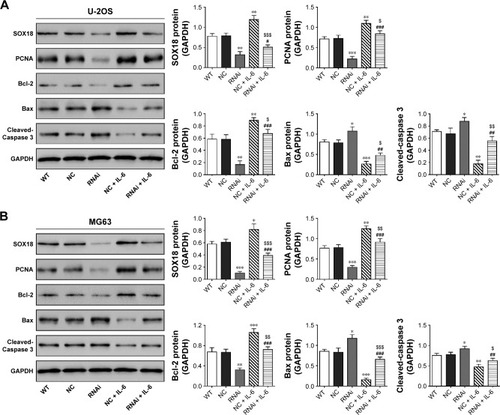
Expression of PCNA, Bcl-2, Bax and Cleaved-Caspase 3
We detected the protein levels of a cell proliferation marker (PCNACitation22) and cell apoptosis-associated proteins (Bcl-2, Bax and Cleaved-Caspase 3Citation23,Citation24). Consistent with the results of CCK-8 and Annexin V-FITC/PI staining assays, IL-6 stimulation enhanced the protein expression of PCNA and Bcl-2, which was reduced by SOX18 knockdown (). The reversed effects were observed in Bax and Cleaved-Caspase 3 expression.
Figure 5 Expression of PCNA, Bcl-2, Bax and Cleaved-Caspase 3.
Abbreviations: WT, without any treatment; RNAi, RNA interference; IL-6, interleukin-6; PCNA, cell proliferation.
Discussion
Our recent study has reported that SOX18 is overexpressed in osteosarcoma and served as a potential oncogene in osteosarcoma via regulating osteosarcoma proliferation and metastasis.Citation11 Several studies have concerned the upstream regulators for SOX genes. For instance, SOX4 was induced by TGF-β in Th2 cells.Citation25 SOX2 expression was induced by STAT3 in the neural precursor cell and breast cancer cells.Citation17,Citation18 IL-6 expression was significantly higher in human osteosarcoma tissues than in the normal bone.Citation26 As a major activator of JAK2/STAT3 signaling,Citation12 IL-6 has been reported to modulate the proliferation of carcinoma cells.Citation19–Citation21 Thus, we tried to investigate the effects of IL-6 treatment on SOX18 expression. In the present study, elevated IL-6 and SOX18 expression were observed in osteosarcoma tissues as compared with bone cyst tissues at both mRNA and protein levels. The increased level of phosphorylated STAT3 is associated with poor prognosis of osteosarcoma.Citation16 Presently, phosphorylated STAT3 was elevated in osteosarcoma tissues. Moreover, SOX18 mRNA expression was strongly correlated with IL-6 expression in osteosarcoma tissues. IL-6 exposure to osteosarcoma cells significantly increased the mRNA and protein expression of SOX18 and the phosphorylation of STAT3 in a dose-dependent manner. Our study suggested that IL-6/STAT3 regulated SOX18 expression in osteosarcoma, although the detailed mechanisms remain to be elucidated.
Furthermore, the involvement of SOX18 in IL-6-affected osteosarcoma cell proliferation and apoptosis was also studied. CCK-8 assays showed that IL-6 treatment significantly promoted osteosarcoma cell proliferation. Knockdown of SOX18 significantly suppressed the effects of IL-6 on cell proliferation. Although IL-6 had little effect on cell apoptosis, it could significantly reduce SOX18 knockdown-induced cell apoptosis. These findings revealed that IL-6 is a possible upstream regulator for SOX18. PCNA is a well-accepted marker for cell proliferation.Citation22 Bcl-2 family proteins can either stimulate cell survival (eg, Bcl-2) or induce cell apoptosis (eg, Bax).Citation23 Cleaved-Caspase 3 is a marker for cell apoptosis.Citation24 Here, the change trends of these four proteins were consistent with the results of CCK-8 and cell apoptosis assays.
Conclusion
IL-6 treatment significantly enhanced SOX18 expression in osteosarcoma. IL-6 rescued the inhibitory and induction effects of SOX18 knockdown on osteosarcoma cell proliferation and apoptosis, respectively. Our study may provide insights to advance our understandings on the occurrence and development of osteosarcoma.
Disclosure
The authors report no conflicts of interest in this work.
References
- OttavianiGJaffeNThe epidemiology of osteosarcomaCancer Treat Res200915231320213383
- FergusonWSGoorinAMCurrent treatment of osteosarcomaCancer Invest200119329231511338887
- HoskingBMMuscatGEKoopmanPADowhanDHDunnTLTrans-activation and DNA-binding properties of the transcription factor, Sox-18Nucleic Acids Res19952314262626287651823
- HoskingBMWyethJRPennisiDJWangSCKoopmanPMuscatGECloning and functional analysis of the Sry-related HMG box gene, Sox18Gene20012621–223924711179689
- DongCWilhelmDKoopmanPSox genes and cancerCytogenet Genome Res20041052–444244715237232
- EomBWJoMJKookMCThe lymphangiogenic factor SOX 18: a key indicator to stage gastric tumor progressionInt J Cancer20121311414821796627
- WangYGuoHZhangDOverexpression of SOX18 correlates with accelerated cell growth and poor prognosis in human pancreatic ductal adenocarcinomaBiochem Biophys Res Commun2016479351051627663663
- PulaBOlbromskiMWojnarAImpact of SOX18 expression in cancer cells and vessels on the outcome of invasive ductal breast carcinomaCell Oncol (Dordr)201336646948324065215
- PulaBKobierzyckiCSolinskiDSOX18 expression predicts response to platinum-based chemotherapy in ovarian cancerAnticancer Res20143484029403725075026
- JethonAPulaBOlbromskiMPrognostic significance of SOX18 expression in non-small cell lung cancerInt J Oncol201546112313225310193
- WuZLiuJWangJZhangFSOX18 knockdown suppresses the proliferation and metastasis, and induces the apoptosis of osteosarcoma cellsMol Med Rep201613149750426573263
- HodgeDRHurtEMFarrarWLThe role of IL-6 and STAT3 in inflammation and cancerEur J Cancer200541162502251216199153
- WilliamsJGSTAT signalling in cell proliferation and in developmentCurr Opin Genet Dev200010550350710980427
- BattleTEFrankDAThe role of STATs in apoptosisCurr Mol Med20022438139212108949
- BrombergJFWrzeszczynskaMHDevganGStat3 as an oncogeneCell199998329530310458605
- RyuKChoyEYangCActivation of signal transducer and activator of transcription 3 (Stat3) pathway in osteosarcoma cells and overexpression of phosphorylated-Stat3 correlates with poor prognosisJ Orthop Res201028797197820063378
- FoshayKMGallicanoGIRegulation of Sox2 by STAT3 initiates commitment to the neural precursor cell fateStem Cells Dev200817226927818447642
- YangJLiaoDChenCTumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathwayStem Cells201331224825823169551
- OkamotoMLeeCOyasuRInterleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitroCancer Res19975711411468988055
- OkamotoMHattoriKOyasuRInterleukin-6 functions as an autocrine growth factor in human bladder carcinoma cell lines in vitroInt J Cancer19977211491549212236
- MikiSIwanoMMikiYInterleukin-6 (IL-6) functions as an in vitro autocrine growth factor in renal cell carcinomasFEBS Lett198925026076102787758
- KubbenFJPeeters-HaesevoetsAEngelsLGProliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferationGut19943545305357909785
- ReedJCRegulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistanceCurr Opin Oncol1995765415468547403
- SekiKYoshikawaHShiikiKHamadaYAkamatsuNTasakaKCisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcomaCancer Chemother Pharmacol200045319920610663637
- KuwaharaMYamashitaMShinodaKThe transcription factor Sox4 is a downstream target of signaling by the cytokine TGF-[beta] and suppresses TH2 differentiationNat Immunol201213877878622751141
- LinY-MChangZ-LLiaoY-YChouM-CTangC-HIL-6 promotes ICAM-1 expression and cell motility in human osteosarcomaCancer Lett2013328113514322939995
