Abstract
Background
Diabetes mellitus (DM) has been identified to be both a risk factor and a prognostic factor in a variety of malignancies, but its association with the risk and outcome of nasopharyngeal carcinoma (NPC) is still unclear. To elucidate this issue, we systematically reviewed the evidence concerning the association between DM status and NPC.
Materials and methods
We identified studies by a literature search of PubMed, Embase, and ISI Web of Knowledge through May 31, 2017, and by searching the reference lists of pertinent articles. Odds ratios (ORs) and hazard ratios (HRs) with 95% CIs were used to estimate the effect size. Heterogeneity across studies was evaluated by the Cochran’s Q and I2 statistics.
Results
A total of nine studies were included. Four studies with a total sample size of 221,611 reported the effect of DM on NPC risk, and the other five studies with a sample size of 9,442 reported the impact of DM on survival in NPC patients. All included studies were retrospective, and mostly conducted in Asian populations. Meanwhile, condition of metformin usage was not considered in all studies. A pooled OR of 0.65 (95% CI: 0.43–0.98, P=0.04) revealed an inverse association between DM and NPC. Additionally, pooled analyses of studies investigating the prognosis value of DM revealed that preexisting DM had no effect on overall survival (HR =1.17, 95% CI: 0.94–1.46, P=0.16), local recurrence-free survival (HR =1.16, 95% CI: 0.80–1.67, P=0.44), and distant metastasis-free survival (HR =1.14, 95% CI: 0.92–1.40, P=0.22).
Conclusion
Our results suggested that DM patients might have decreased NPC risk, and have little impact on prognosis of NPC patients. This conclusion should be limited to Asian population. Our results also suggest that more attention should be paid to metformin medication in further studies in order to clarify whether the effects of DM on NPC risk and prognosis are influenced by the anticancer effect of metformin.
Introduction
The relationship between malignancies and diabetes mellitus (DM) has become a critically important area of study because of the concomitant increase in the incidence of DM.Citation1 Epidemiological studies suggest that individuals with DM have an elevated risk of various types of cancers, such as hepatocellular carcinoma, cholangiocarcinoma, pancreatic duct adenocarcinoma, gastric cancer, colorectal cancer, renal carcinoma, bladder cancer, and breast cancer.Citation2–Citation7 Additionally, DM has been identified to be an adverse prognostic factor for various kinds of cancers, and many studies identified that cancer patients with preexisting diabetes are at increased risk for poor prognosis compared with those without diabetes.Citation8,Citation9 However, in contrast to the findings in most cancer types, DM appeared to have no impact on the prognosis of head and neck cancer,Citation10 and a study even found that patients with DM had a weakly decreased risk of head and neck squamous cell cancer.Citation11
Nasopharyngeal carcinoma (NPC) is a unique type of head and neck cancer, with a low incidence rate below 2 per 100,000 person-years in western countries.Citation12 Conversely, NPC shows a particularly high incidence in Southeast Asia and its surrounding regions.Citation13 NPC demonstrates distinct epidemiology, etiology, pathophysiology, clinical characteristics, and therapeutic model in comparison with other cancers, including other squamous cell carcinomas of the head and neck.Citation14 Studies also reported controversial results about the relationship between DM and NPC.Citation15–Citation23 Therefore, the present study aimed to summarize results from relevant studies, and to provide insight into the relationship between DM and NPC.
Materials and methods
Search and filtration strategy
A systematic literature search of PubMed, Embase, and ISI Web of Knowledge was conducted to retrieve clinical studies up to May 2017. We used MeSH terms and text words related to both the exposure (diabetes, diabetes mellitus, blood glucose, hyperglycemia, and impaired glucose tolerance) and populations (nasopharynx cancer, nasopharynx carcinoma, nasopharynx neoplasm, nasopharyngeal cancer, nasopharyngeal carcinoma, nasopharyngeal neoplasm, pharyngeal cancer, pharyngeal carcinoma, and pharyngeal neoplasm) to search for related articles. The language of all publications was limited to English only. The initial selection was performed to exclude obviously irrelevant articles and retain potentially relevant articles about the effect of DM on NPC risk and/or outcome by an analysis of the title and abstract by two independent investigators (G Guo and M Fu). Thereafter, the full text was reviewed according to the including criteria: 1) prospective and retrospective studies that researched the relationship between DM and NPC risk; 2) one of the following risk indexes, including odds ratio (OR), relative risk (RR), rate difference, or attributable risk, along with the 95% CIs or P-values should be available. The following publications were excluded: duplicated literatures, duplicated reported data, letters, reviews, expert opinions, or case reports.
Data extraction and quality assessment
Two investigators (G Guo and M Fu) independently extracted data from the full manuscript independently using a standardized form. The following items were collected from each study: first author, year of publication, study design, geographic areas of the study population, sample size, age and sex of patients, follow-up time, statistic model, and outcome measurements. For assessing the impact of DM on NPC risk, OR was preferred as the primary outcome. The hazard ratio (HR) was preferred for evaluating the survival outcome because it is time-to-event data. For studies that reported only survival curves, the HR values were obtained by contacting the authors or were estimated by the methods described by Tierney et al.Citation24 Multivariate outcomes were used for meta-analyses, but univariate outcomes were used instead if no multivariate results were presented. The quality assessment of included studies was independently applied using the “Newcastle–Ottawa Scale (NOS)”, which includes three domains with eight items. Each item could be awarded 1 to a maximum of 2 score, and the total possible score was 9. Study with a score ≥6 was deemed as being of good quality.
Statistical analysis
All meta-analyses were performed using Review Manager software (Version 5.3; The Cochrane Collaboration, Copenhagen, Denmark). The heterogeneity of the included studies was evaluated using the Cochran’s Q-test and Higgins I2 statistic. Pooled analysis with a P≥0.10 or I2≤50% was considered to have low heterogeneity, and a fixed-effects model was subsequently applied. Otherwise, the random-effects model was applied for meta-analysis. Additionally, the funnel plot was used to evaluate the publication bias. A two-tailed P<0.05 was considered statistically significant. All the results are presented in the forest plots.
Results
shows the flow chart for study selection. Initially, 296 articles were retrieved using the search words, of which 80 were duplicates. After title and abstract screening, 65 studies were involved in the full-text review. Studies that did not report NPC risk or survival information, and those that were basic researches, reviews, case reports, commentaries, or meta-analyses were excluded from our analysis. The reference lists of retrieved reviews and meta-analyses were also examined for potential relevant studies, but no more articles were identified. After further full-test review, 56 articles were excluded and nine studies were ultimately included in the present meta-analysis,Citation15–Citation23 of which four studies reported association between DM and NPC risk (),Citation15–Citation18 and another five studies reported association between DM and NPC outcome ().Citation19–Citation23
Table 1 Characteristics of included studies investigating the relationship between DM and NPC risk
Table 2 Characteristics of included studies investigating the impact of DM on NPC prognosis
Figure 1 Literature screening flowchart.
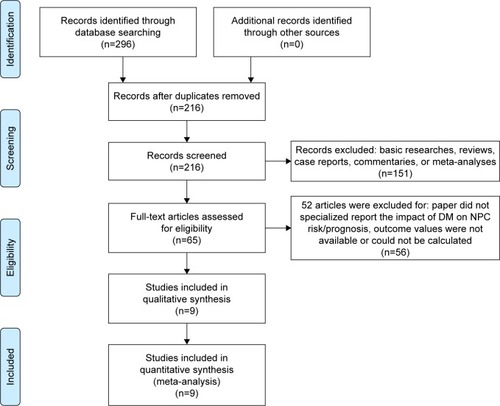
Association between DM and NPC risk
The baseline characteristics of the included studies reporting association between DM and NPC risk are summarized in . A total of 221,611 patients were included. All studies were retrospectively designed. Two studies were published in 2014, and the other two studies were published in 2016. Three studies were conducted in Southeast Asia, and one in Europe. Matched design was introduced in two studies,Citation16,Citation18 and three studies used multivariate analysis to calculate ORs.Citation16–Citation18 Hsieh et al reported ORs from three subgroups instead of the whole cohort.Citation15 Based on the NOS, the quality scores of each study were more than 5, ranging from 6 to 8. The lack of comparability between groups was found in two studies.Citation15,Citation17 The study of Tseng et al did not exclude potential cancer patients in the control group at the initial time of identifying patients.Citation16
To evaluate the relationship between DM and NPC risk, we pooled the results of four relevant studies (). We noted that Hsieh et al reported three RR values from three subgroups;Citation15 because each of the three subgroups was from a different and independent population, we put them into the meta-analysis as three independent studies. The heterogeneity test showed that major heterogeneity exists (I2=85%) among these studies, and thus a random-effects model was used for the analysis. A pooled OR of 0.65 (95% CI: 0.43–0.98, P=0.04) revealed an inverse association between DM and NPC. Meta-analysis of three studies conducted in Asia also showed an inverse association between DM and NPC (OR =0.62, 95% CI: 0.39–0.97, P=0.04) ().Citation15–Citation17 The above results suggest that DM appears to be correlated with a trend toward decreased NPC risk, which is quite different from the finding that DM is positively associated with an increased risk of most other cancers.
Figure 2 Forest plots for the association between diabetes mellitus and nasopharyngeal carcinoma.
Abbreviations: DM, diabetes mellitus; NPC, nasopharyngeal carcinoma.
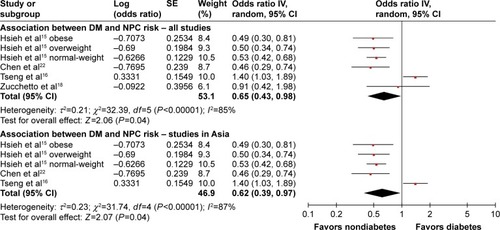
Association between DM and NPC outcome
Five studies reported the influence of DM on outcomes among patients with NPC.Citation19–Citation23 The main characteristics of these five included studies are listed in . The included studies were published between 2006 and 2017. All studies were conducted in Asian population, and 9,442 NPC cases were included. All studies retrospectively analyzed data. Three studies reported the median follow-up period,Citation20,Citation21,Citation23 with a range between 34.6 and 66.0 months. Four studies adopted multivariate analysis method,Citation19–Citation22 but only one study used a matched design to select control patients.Citation20 DM was identified in the cohorts using blood glucose test results validated by medical records in all five studies.
To investigate the impact of DM on prognosis of NPC patients, three outcome measurements, including overall survival (OS), local recurrence-free survival (LRFS), and distant metastasis-free survival (DMFS), were quantitatively pooled. The meta-analysis results are displayed in . Heterogeneity is illustrated in each forest plot. OS outcomes were available from all five studies, and the pooled result did not show significantly decreased OS in NPC patients with DM (HR =1.17, 95% CI: 0.94–1.46, P=0.16, I2=35%). Four studies provided sufficient data on LRFS outcome. Pooled results did not show a significantly higher risk of tumor local recurrence in patients with DM (HR =1.16, 95% CI: 0.80–1.67, P=0.44, I2=69%). Similarly, four studies provided DMFS information, and the DM history and DMFS were not found to be significantly associated in the pooled analysis (HR =1.14, 95% CI: 0.92–1.40, P=0.22, I2=0%). In summary, all of the meta-analysis results found no significant differences of OS, LRFS, and DMFS when comparing patients with diabetes to those with normoglycemia.
Figure 3 Forest plot for the impact of diabetes mellitus on the prognosis of nasopharyngeal carcinoma patients.
Publication bias
Funnel plots were introduced for estimating the publication bias. The shape of the funnel plots () seemed unsymmetrical, suggesting that there was a potential publication bias. But due to the less number of studies selected in our meta-analysis, the funnel plot is of less significance.
Figure 4 Funnel plot analysis of the included articles’ publication bias about diabetes mellitus and nasopharyngeal carcinoma.
Abbreviation: OR, odds ratio.
Discussion
Numerous studies have identified close associations between DM and a variety of cancers in various populations, suggesting that DM appears to be a risk factor for various kinds of cancers, and patients diagnosed with cancer who have preexisting diabetes are at increased risk for long-term, all-cause mortality compared with those without diabetes.Citation25–Citation29 The potential link between DM and cancer has been hypothesized to be related to insulin, insulin-like growth factor, inflammatory status, metabolic characteristics, and even certain treatments of the DM.Citation30,Citation31 High levels of insulin (including the use of exogenous insulin) and IGF-1 can activate receptors and the downstream pathways associated with cell proliferation and subsequently increase cancer risk and promote cancer development.Citation32–Citation34 Other factors and potential mechanism involved chronic subclinical inflammation, abnormal carbohydrate and lipid metabolism, abnormalities in sex hormone metabolism, and excessive activation of Akt/mammalian target of rapamycin and Wnt/Beta-catenin pathways.Citation35–Citation37 However, in contrast to the relationship between DM and most cancers, our present study showed an inverse association between DM and NPC, and found that the diabetic NPC patients had prognosis similar to non-DM NPC patients.
Of all the included studies concerning the association between DM and NPC risk, only Tseng et al found that DM is associated with an increased risk of developing NPC;Citation16 however, the study was limited by high risk of selection bias. They did not exclude cancer patients while selecting study population. In of their manuscript, the cumulative incidence curve of cancer in patients with DM showed a sharply rising curve at the time the study began, which suggested that there were more preexisting cancer patients in the DM cohort than in the non-DM cohort. Besides, the regular medical visits in patients with DM might have increased the chances of an early diagnosis of cancer. The negative association between diabetes and risk of head and neck cancer from the prior reports,Citation11 along with the inverse relationship between diabetes and development of larynx cancer,Citation3,Citation38 also indirectly suggested that DM could not increase NPC risk.
NPC is a unique type of head and neck cancer, which exhibits distinct endemic distribution, close association with Epstein–Barr virus, and relative sensitivity to radiation and chemotherapy in contrast to other head and neck malignancies.Citation13 For example, in high-incidence areas, undifferentiated NPC is the most frequent histological subtype, and differentiated cases are extremely rare, while in western countries, differentiated NPC cases were common and can make up to 25% of all NPCs.Citation39–Citation41 The peculiar geographic distribution of NPC reflects the differences in NPC subtypes and epidemiological patterns of known risk factors. In the study conducted in Italy, Zucchetto et al reported that the prevalence of DM in differentiated NPC patients was 8.7% compared to a rate of 4.4% for the undifferentiated cases;Citation18 they also concluded that metabolic disorders could increase the risk of differentiated NPC, but not undifferentiated NPC. Therefore, as most included studies were conducted in Southeast Asia where differentiated NPC was rare, whether the negative pooled result was due to histology distribution needs further study.
Many studies have reported a variety of potential mechanisms linking diabetes to carcinogenic processes in various kinds of malignancies.Citation30 So far, five studies have examined cancer-specific mortality among patients with NPC with or without diabetes.Citation19–Citation23 Our current meta-analysis of the present five studies did not find a clear correlation between poor prognosis of patients with NPC and DM. Overall, with respect to mortality among NPC patients with or without DM, only Chen et al reported that the diabetes group had a significantly increased mortality rate than the non-DM group.Citation22 All of the other four studies reported that the diabetic NPC patients had similar OS rate to normoglycemic patients.Citation19–Citation21,Citation23 We noted that, in the study of Chen et al,Citation22 the follow-up time was not reported, and the mortality rate of diabetic NPC patients in their cohort was 18%, which was the highest among all cancer types in their cohort, even higher than the mortality rate (14%) of pancreatic cancer patients reported in their cohort. These questioned their results. Besides, regarding the LRFS and DMFS, Peng et al reported that the DM group had a worse LRFS than the non-DM, but they found that the DM NPC patients had similar OS and DMFS to non-DM NPC patients. Other studies also did not find that DM had prognostic impact on LRFS or DMFS of NPC patients.
The above inconsistent results among the included studies of our present meta-analysis may be due to the difference in the sample sizes, selection bias, and the confounding factors caused by the retrospective nature. Additionally, the use of metformin, an insulin sensitizer from the family of the biguanides, was not considered in all studies. Metformin has been widely used in the treatment of DM for decades and is used as a first-line therapy in type 2 diabetes.Citation42 Recently, its anticancer potential has also been discovered. Metformin shows inhibitory effect on some pathways that play an important role in cancer cell proliferation and angiogenesis, thereby inhibiting cancer cell growth and development.Citation43–Citation47 Emerging evidences showed that the use of metformin in cancer patients is related to a survival benefit.Citation48–Citation51 Therefore, whether the oncogenic effect of DM on NPC was diminished by metformin medication, and thus induced decreased risk of NPC in DM population, and a similar survival among NPC patients with and without DM need further study. Another limitation of the currently available studies on the topic of association between DM and NPC risk is that they rarely differentiated type 1 DM and type 2 DM, which may have an effect on the results as type 1 diabetes and type 2 diabetes have totally different pathogenesis. Due to this limitation, the present meta-analysis for DM and NPC risk also cannot perform a separate analysis for type 1 DM and type 2 DM. It is very necessary to differentiate type1 DM and type 2 DM in future studies.
DM is a chronic disease that can cause hyperglycemia-related comorbidities such as hypertension, hyperlipidemia, nerve damage, and cardiovascular complications.Citation52 It has been established that amount and degree of comorbidities significantly affect the prognosis of NPC patients.Citation53,Citation54 Interestingly, Kiderlen et al found that patients with diabetes without other comorbidity had a similar OS as patients without any comorbidity.Citation55 Peng et al found that patients with diabetes-related hyperlipidemia exhibited poor physical condition, resulting in poor prognosis, while diabetes did not have impact on survival.Citation19 These findings suggest that the additional comorbidity in patients with diabetes, but not the diabetes itself, plays a major role in affecting the survival of patients. More attention should be paid to diabetes-related comorbidities, both in clinical management of NPC and in further studies involving the effect of diabetes on survival of NPC patients.
Conclusion
In our current study, diabetes was found to have neither significant impact on NPC risk nor clear association with survival of NPC patients. This conclusion should be limited to Asian population. Our results also suggest that more attentions should be paid to metformin medication in further studies in order to clarify whether the effects of DM on NPC risk and prognosis are influenced by the anticancer effect of metformin.
Disclosure
The authors report no conflicts of interest in this work.
References
- VinerRWhiteBChristieDType 2 diabetes in adolescents: a severe phenotype posing major clinical challenges and public health burdenLancet2017389100852252226028589895
- La VecchiaCNegriEFranceschiSD’AvanzoBBoylePA case-control study of diabetes mellitus and cancer riskBr J Cancer19947059509537947103
- BosettiCRosatoVPoleselJDiabetes mellitus and cancer risk in a network of case-control studiesNutr Cancer201264564365122519904
- RousseauMCParentMEPollakMNSiemiatyckiJDiabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, CanadaInt J Cancer200611882105210916284945
- O’MaraBAByersTSchoenfeldEDiabetes mellitus and cancer risk: a multisite case-control studyJ Chronic Dis19853854354413998058
- SasazukiSCharvatHHaraAResearch Group for the Development and Evaluation of Cancer Prevention Strategies in JapanDiabetes mellitus and cancer risk: pooled analysis of eight cohort studies in JapanCancer Sci2013104111499150723889822
- ShikataKNinomiyaTKiyoharaYDiabetes mellitus and cancer risk: review of the epidemiological evidenceCancer Sci2013104191423066889
- GallagherEJLeRoithDObesity and diabetes: the increased risk of cancer and cancer-related mortalityPhysiol Rev201595372774826084689
- BaroneBBYehHCSnyderCFLong-term all-cause mortality in cancer patients with preexisting diabetes mellitus: a systematic review and meta-analysisJAMA2008300232754276419088353
- BianchiniCCiorbaAAimoniCHead and neck cancer patients: impact of diabetes mellitus on surgical outcomesJ BUON201621358058727569076
- Stott-MillerMChenCSchwartzSMType II diabetes and metabolic syndrome in relation to head and neck squamous cell carcinoma risk: a SEER-Medicare database studyCancer Epidemiol201337442843323562170
- PeterssonFNasopharyngeal carcinoma: a reviewSemin Diagn Pathol2015321547325769204
- ChuaMLWeeJTHuiEPChanATNasopharyngeal carcinomaLancet2016387100221012102426321262
- CaponigroFLongoFIonnaFPerriFTreatment approaches to nasopharyngeal carcinoma: a reviewAnticancer Drugs201021547147720124988
- HsiehSHChiouWKWangMHLinJDAssociation of body weight with the risk for malignancies in hospitalized patients with or without diabetes mellitus in TaiwanJ Investig Med20146213742
- TsengKSLinCLinYSWengSFRisk of head and neck cancer in patients with diabetes mellitus: a retrospective cohort study in TaiwanJAMA Otolaryngol Head Neck Surg2014140874675325058016
- RoujunCYanhuaYBixunLHigh prevalence of diabetes mellitus and impaired glucose tolerance in liver cancer patients: a hospital based study of 4610 patients with benign tumors or specific cancersF1000Res20165139727610222
- ZucchettoATaborelliMBosettiCMetabolic disorders and the risk of nasopharyngeal carcinoma: a case-control study in ItalyEur J Cancer Prev Epub2016729
- PengHChenLZhangYPrognostic value of diabetes in patients with nasopharyngeal carcinoma treated with intensity-modulated radiation therapySci Rep201662220026927312
- PengXSXieGFQiuWZTianYHZhangWJCaoKJType 2 diabetic mellitus is a risk factor for nasopharyngeal carcinoma: a 1:2 matched case-control studyPLoS One20161110e016513127760202
- OuYangPYSuZTangJDiabetes, prediabetes and the survival of nasopharyngeal carcinoma: a study of 5,860 patientsPLoS One2014910e11107325350747
- ChenJYChiouWKChouWYLinJDThe impact of type 2 diabetes mellitus on mortality in hospitalized female cancer patients in TaiwanAsia Pac J Clin Oncol2016121e75e8123714100
- LiuHXiaYCuiNImpact of diabetes mellitus on treatment outcomes in patients with nasopharyngeal cancerMed Oncol200623334134617018891
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582
- GiovannucciEHarlanDMArcherMCDiabetes and cancer: a consensus reportDiabetes Care20103371674168520587728
- ZhuBWuXWuBPeiDZhangLWeiLThe relationship between diabetes and colorectal cancer prognosis: a meta-analysis based on the cohort studiesPLoS One2017124e017606828423026
- ZhuLCaoHZhangTThe effect of diabetes mellitus on lung cancer prognosis: a PRISMA-compliant meta-analysis of cohort studiesMed20169517e3528
- ZhouYZhangXGuCXiaJDiabetes mellitus is associated with breast cancer: systematic review, meta-analysis, and in silico reproductionPanminerva Med201557310110825971328
- WangYGWangPWangBFuZJZhaoWJYanSLDiabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysisPLoS One201495e9548524830459
- GallagherEJLeRoithDEpidemiology and molecular mechanisms tying obesity, diabetes, and the metabolic syndrome with cancerDiabetes Care201336Suppl 2S233S23923882051
- ShlomaiGNeelBLeRoithDGallagherEJType 2 diabetes mellitus and cancer: the role of pharmacotherapyJ Clin Oncol201634354261426927903154
- AhmadiehHAzarSTType 2 diabetes mellitus, oral diabetic medications, insulin therapy, and overall breast cancer riskISRN Endocrinol2013201318124023401790
- MillerBSYeeDType I insulin-like growth factor receptor as a therapeutic target in cancerCancer Res20056522101231012716287993
- IbrahimYHYeeDInsulin-like growth factor-I and breast cancer therapyClin Cancer Res2005112 Pt 2944s950s15701891
- Ali KamkarMMAhmadRAlsmadiOBehbehaniKInsight into the impact of diabetes mellitus on the increased risk of hepatocellular carcinoma: mini-reviewJ Diabetes Metab Dis20141357
- YangJNishiharaRZhangXOginoSQianZREnergy sensing pathways: bridging type 2 diabetes and colorectal cancer?J Diabetes Complications20173171228123628465145
- LecarpentierYClaesVValleeAHebertJLInteractions between PPAR Gamma and the Canonical Wnt/Beta-Catenin Pathway in Type 2 Diabetes and Colon CancerPPAR Res20172017587909028298922
- KoSYoonSJKimDKimARKimEJSeoHYMetabolic risk profile and cancer in Korean Men and WomenJ Prev Med Public Health201649314315227255073
- FaivreSJanotFArmandJPOptimal management of nasopharyngeal carcinomaCurr Opin Oncol200416323123515069318
- SanguinetiGCorvoRTreatment of nasopharyngeal carcinoma: state of the art and new perspectives (review)Oncol Rep19996237739110023009
- LeeAWMaBBNgWTChanATManagement of nasopharyngeal carcinoma: current practice and future perspectiveJ Clin Oncol201533293356336426351355
- Sanchez-RangelEInzucchiSEMetformin: clinical use in type 2 diabetesDiabetologia20176091586159328770321
- StorozhukYHopmansSNSanliTMetformin inhibits growth and enhances radiation response of non-small cell lung cancer (NSCLC) through ATM and AMPKBr J Cancer2013108102021203223632475
- GrissTVincentEEEgnatchikRMetformin antagonizes cancer cell proliferation by suppressing mitochondrial-dependent biosynthesisPLoS Biol20151312e100230926625127
- KatoKGongJIwamaHThe antidiabetic drug metformin inhibits gastric cancer cell proliferation in vitro and in vivoMol Cancer Ther201211354956022222629
- BaoBWangZAliSMetformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cellsCancer Prev Res (Phila)20125335536422086681
- GuiDYSullivanLBLuengoAEnvironment dictates dependence on mitochondrial complex I for NAD+ and aspartate production and determines cancer cell sensitivity to metforminCell Metab201624571672727746050
- WhitburnJEdwardsCMSooriakumaranPMetformin and prostate cancer: a new role for an old drugCurr Urol Rep20171864628444639
- GarciaCYaoACamachoFBalkrishnanRCantrellLAA SEER-Medicare analysis of the impact of metformin on overall survival in ovarian cancerGynecol Oncol2017146234635028499649
- Perez-LopezFRPasupuletiVGianuzziXPalma-ArdilesGHernandez-FernandezWHernandezAVSystematic review and meta-analysis of the effect of metformin treatment on overall mortality rates in women with endometrial cancer and type 2 diabetes mellitusMaturitas201710161128539171
- MartinMMaraisRMetformin: a diabetes drug for cancer, or a cancer drug for diabetics?J Clin Oncol201230212698270022565000
- JelinekHFOsmanWMKhandokerAHClinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab EmiratesBMJ Open Diabetes Res Care201751e000427
- GuoRChenXZChenLComorbidity predicts poor prognosis in nasopharyngeal carcinoma: development and validation of a predictive score modelRadiother Oncol2015114224925625618213
- GuoRMaoYPChenLImplication of comorbidity on the initiation of chemotherapy and survival outcomes in patients with locoregionally advanced nasopharyngeal carcinomaOncotarget201786105941060127070084
- KiderlenMde GlasNABastiaannetEDiabetes in relation to breast cancer relapse and all-cause mortality in elderly breast cancer patients: a FOCUS study analysisAnn Oncol201324123011301624026538
