Abstract
Background
Systemic inflammation can be reflected by peripheral hematologic parameters and combined index like the lymphocyte count, neutrophil count, platelet count, neutrophil-to-lymphocyte (NLR), and platelet-to-lymphocyte ratio (PLR). This systematic review and meta-analysis aimed to summarize the association between the hematologic markers and prognosis of gastroenteropancreatic neuroendocrine tumors (GEP–NETs).
Methods
A computerized systematic search of PubMed, Embase, and Web of Science was conducted up to August 2016. Studies evaluating prognosis value of hematologic parameters in patients with GEP–NETs were retrieved. For meta-analysis, hazard ratios (HRs) with 95% confidence intervals (95% CIs) were extracted and synthesized using Review Manager software.
Results
We identified eight retrospective cohort studies comprising a total of 724 cases. The majority of included studies focused on pancreatic neuroendocrine tumors (PNETs). The prognostic values of NLR, PLR, and platelet count were reported in six studies, two studies, and one study, respectively. All the parameters were associated with prognostic outcomes in patients with GEP–NETs. A high NLR was significantly associated with poor prognosis in GEP–NETs (pooled HR 3.05, 95% CI 1.96–4.76, I2 = 0%, P < 0.00001 for overall survival (OS); pooled HR 3.30, 95% CI 2.04–5.32, I2 = 0%, P < 0.00001 for recurrence-free survival [RFS]). In PNETs, pooled-analyses also showed significant superiority of a low NLR on OS (pooled HR 4.21, 95% CI 1.95–9.13, I2 = 0%, P = 0.0003) and RFS (pooled HR 5.37, 95% CI 2.14–13.47, I2 = 0%, P = 0.003).
Conclusions
These findings suggest that the elevated NLR could be an adverse prognosis factor for GEP–NETs. The conclusion should be mainly limited to PNETs as the majority of included cases were PNET patients. The prognostic value of other hematologic parameters deserves further investigation. We recommend that further studies should use a continuous NLR variable and adopt a prospective and matched study design.
Introduction
Gastroenteropancreatic neuroendocrine tumors (GEP–NETs) are biologically diverse neoplasms that arise from the diffuse endocrine system in the gastrointestinal tract and/or pancreas.Citation1 In recent decades, GEP–NETs have exhibited a significantly increased incidence,Citation2 and today comprise approximately 2% of all malignant gastrointestinal tumors.Citation3 Due to the highly heterogeneous features and unpredictable biological behaviors of GEP–NETs, discovery of markers with efficient diagnosis and/or prognosis effect could help determine optimal clinical managements and follow-up strategies.Citation4–Citation7
The link between chronic inflammation and cancer has been established for a long time.Citation8,Citation9 Numerous epidemiologic studies support a clear connection between chronic inflammation and the development of many cancers. In turn, the tumor itself can initiate and maintain inflammatory processes that foster tumor growth and development. Many inflammation-related cytokines and chemokines have been extensively documented in cancers of the stomach, liver, lung, esophagus, breast, and prostate. In addition, an important hallmark of cancer is that cancer cells evade immunological attack, and recent studies have identified that chronic inflammation is associated with immunosuppression, mediated primarily by immature myeloid-derived suppressor cells.Citation10–Citation12
Studies in the past decades have identified a close connection between GEP–NETs and chronic inflammation. It was shown that chronic inflammation can lead to hyperplasia and neoplastic transformation of enteroendocrine cells.Citation13–Citation15 Additionally, single nucleotide polymorphisms of some inflammatory cytokines, such as TNF-α −1031T/C, IL-6 −174 C/G, and IL-2 −330T/G allele, have been identified to be associated with the overall susceptibility to develop GEP–NETs.Citation16–Citation18 Nowadays, many typical factors of systematic inflammation, such as C-reactive protein, interleukin, some growth factors and chemokines have been validated as predictive in various types of cancer.Citation19–Citation21 Recently, emerging studies have focused upon the prognosis value of hematologic parameters of systemic inflammation, including leukocyte counts, neutrophil counts, platelet counts, and the ratios between them such as neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) because they are cheap and easily acquired markers during clinical practice.Citation22–Citation26 Hence, we aimed to conduct a systematic review and meta-analysis to evaluate the prognostic value of hematologic parameters in patients with GEP–NETs.
Methods
Study identification and selection
A systematic literature search of PubMed, Embase, and the Institute for Scientific Information (ISI) Web of Knowledge was conducted in August, 2017, by two independent reviewers (Yu Zhou & Dezhi Li) to retrieve potential relevant studies with restriction to English language. We used Mesh terms and text words to retrieve potential eligible studies with the following retrieval logic: (“neutrophil” or “lymphocyte” or “leukomonocyte” or “monocyte” or “platelet” or “thrombocyte” or “blood cell” or “blood routine” or “hematologic” or “hematological”) and (“neuroendocrine tumor” or “neuroendocrine tumour” or “neuroendocrine neoplasm” or “neoplasm cancer” or “neoplasm malignancy” or “carcinoid” or “insulinoma” or “vipoma” or “gastrinoma” or “paraganglioma”). We did not limit the search based on tumor site of gasteoenteropancreatic organs in case of missing articles. Reference lists of the retrieved articles were also searched for relevant studies. The initial selection was performed to eliminate obviously irrelevant articles, reviews, meeting abstracts, comments, letters, and basic research. We retained potentially relevant articles about hematologic parameters or GEP–NET prognostic risk factors by reviewing the titles and abstracts. Thereafter, the full-text was reviewed. Studies of patients with GEP–NETs that evaluated the effect of at least one of the hematologic parameters on prognosis were included.
Data extraction and quality assessment
The investigators (Yu Zhou & Dezhi Li) extracted information independently using a standardized data extraction table. The information about the basic characteristics of included studies and population, details of methodological characteristics, and relevant outcomes were recorded, including first author, year of publication, study period, study design, sample size, clinicopathologic characters of the study cohort, cut-off value of hematologic parameters, method of statistical analysis, and clinical outcomes. The hazard ratio (HR) was preferred for evaluating the survival outcome since it is time-to-event data. The values of HRs, 95% CIs, and P-values were extracted. For studies that did not provide sufficient data, the HR values were obtained by contacting the corresponding authors or were estimated by the methods described by Tierney et al.Citation44 The primary outcomes were cancer-specific survival (CSS) and overall survival (OS). The secondary outcomes included recurrence-free survival (RFS), distal metastasis-free survival (DMFS), local relapse-free survival (LRFS), and progression-free survival (PFS). There are no standard quality-assessment tools for prognostic studies in systematic reviews. The Newcastle-Ottawa Quality Assessment Scale (NOS) was adopted to assess the quality of each included study independently by our two investigators. NOS scores more than 6 were considered as high-quality studies. The two investigators had discussions to reach a consensus when there was any disagreement.
Statistical analysis
All the synthesis analyses were carried out using the Review Manager software (Version 5.3, The Cochrane Collaboration, Copenhagen, Denmark). A two-tailed P-value < 0.05 was considered statistically significant. HRs with 95% CIs were used to evaluate the prognosis value of hematological parameters (high level vs low level). When the study reported both univariate and multivariate results, we chose multivariate analysis for final calculation. Cochran’s Q test and Higgins I2 statistic were performed for evaluating heterogeneity among studies. Studies with a P ≥ 0.1 or I2 < 50% were considered to have low heterogeneity and the fixed-effects model was used. Otherwise, the random-effects model was applied. A funnel plot was performed to assess publication bias.
Ethics approval
Since this was a protocol for a systematic review based upon available evidence, ethics approval was not required.
Results
Data retrieval
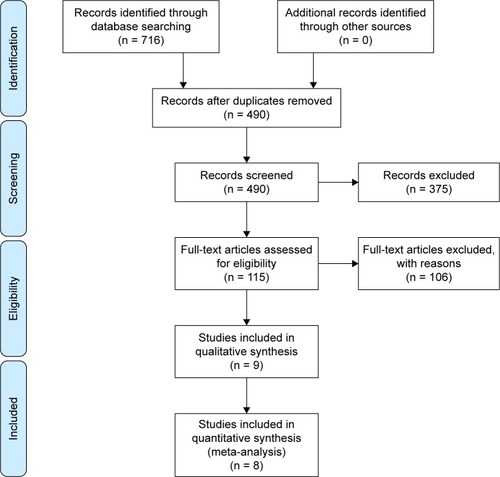
shows the flow chart for study search and selection. After searching the database of PubMed, Embase, and ISI Web of Knowledge, we finally identified 716 relevant references, of which 226 were duplicated. After removing duplicate articles, and further screening titles and abstracts, 389 articles were excluded, including laboratory investigations, case reports, meeting abstracts, comments, letters, reviews, and other articles irrelevant to our topic. After the full-text review, a total of eight studies were ultimately included.
Characteristics of studies and data quality
and show the characteristics of included studies. The eight eligible studies were published between 2009 and 2017, and all were retrospective analysis.Citation27–Citation34 A total of 724 cases were involved, and the sample sizes of included studies ranged from 34 to 165. Five studies only enrolled PNETs patients,Citation27–Citation30,Citation34 one study only enrolled patients with gastric neuroendocrine tumors (G–NETs),Citation31 one study enrolled patients with gastro-entero-pancreatic neuroendocrine tumors (GEP–NETs),Citation32 and another study enrolled patients with neuroendocrine tumors regardless of the primary site.Citation33 The prognosis values of NLR were reported in six articles,Citation27–Citation29,Citation31–Citation33 the prognosis values of PLR were reported in two articles,Citation34 and only one study reported the prognosis value of platelet count.Citation30 Most studies determined the cut-off values of the hematologic markers by using receiver operating characteristic (ROC) curves to select the most significant points. The HRs for survival outcomes were provided in seven studies.Citation27–Citation31,Citation33,Citation34 and all of them were adjusted for potential confounders using the Cox proportion hazard model. The NOS scores of the included studies ranged from 6 to 7. The major inadequacies among the included studies were incomparability between groups.
Table 1 Basic characteristics of included studies
Table 2 Methodology characters of included studies
Correlation between hematologic parameters and survival outcomes
NLR
summarizes the results of the prognostic value of each hematologic parameter. Most studies focused on the prognosis value of NLR. The effect of NLR on OS, RFS, and LMFS was available in four studies,Citation27,Citation28,Citation31,Citation33 four studies,Citation27,Citation29,Citation31,Citation32 and one study,Citation27 respectively. All of these studies suggested that NLR was a marker for poor prognosis. Using the Cox proportional-hazard model, four studies showed patients with high NLR had poor OS,Citation27,Citation28,Citation31,Citation33 and three studies reported that high NLR correlated with poor RFS.Citation27,Citation29,Citation31 Arima et al also specifically reported NLR was an independent predictor of postoperative liver metastasis.Citation27 In addition, Salman et al found that a median NLR of 2.17 accurately predicted a PFS of 11.5 months (area under the curve [AUC] 0.94, P < 0.001) with 98.5% sensitivity and 53.7% specificity on the ROC curve.Citation32 Pooled-analyses of the HRs revealed that patients with elevated NLR had both higher mortality risk and recurrence risk than those with a low NLR (pooled HR 3.05, 95% CI 1.96–4.76, I2 = 0%, P < 0.00001 for OS; pooled HR 3.30, 95% CI 2.04–5.32, I2 = 0%, P < 0.00001 for RFS).
Table 3 Reported outcomes in each study
PLR
Two studies reported the effect of PLR on prognosis.Citation32,Citation34 The study conducted by Sakka et al found that decreased PLR values predicted better OS thorough the Kaplan–Meier method and survival curves.Citation34 Salman et al showed patients with lower PLR values had decreased median PFS time in comparison with patients with higher PLR values, and additionally they also revealed that a median PLR of 181.5 accurately predicted a PFS of 12.5 months by using the ROC analysis.Citation32 Because the study by Sakka et al only reported HR value, pooled-analysis was not performed.Citation34 The results of these two studies suggested lower PLR predicted better prognosis, but more evidence is needed.
P count
Only one study reported the prognosis value of platelet count. The study by Kaltenborn et al found the OS was significantly different between patients with low platelet counts and those with high platelet counts and showed that patients with higher platelet counts had better OS.Citation30
NLR in PNET
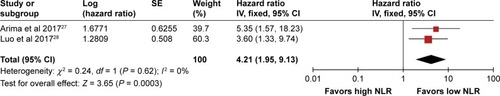
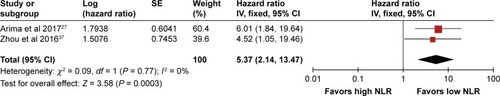
Five studies enrolled only PNET patients. Among them, two studiesCitation27,Citation28 provided sufficient data on OS,Citation27–Citation30,Citation34 and two studies provided sufficient data on RFS outcome for the pooled estimate.Citation27,Citation29 As shown in , the result of meta-analysis showed significant superiority of a low NLR on OS (pooled HR 4.21, 95% CI 1.95–9.13, I2 = 0%, P = 0.0003). Meanwhile, the pooled HR for RFS also favored patients with a low NLR (pooled HR 5.37, 95% CI 2.14–13.47, I2 = 0%, P = 0.003) ().
Publication bias
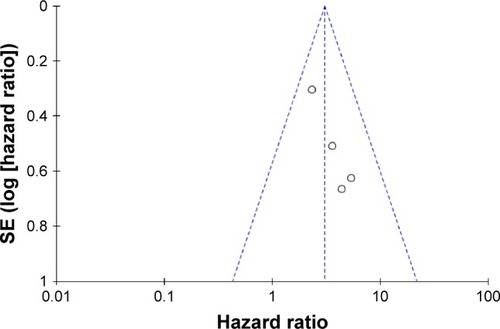
The funnel plot showed an unsymmetrical distribution around the vertical axis (). However, because the number of included studies was small, the funnel plots may make little sense.
Discussion
Several hematologic parameters of systemic inflammation, such as lymphocyte counts, neutrophil counts, platelet counts, NLR, LMR, and PLR, have emerged as prognostic factors for a variety of cancer types.Citation35–Citation38 Because these markers can be easily acquired from blood routine test, they are cheap and easily acquired prognostic markers with a potential for widespread clinical use, many studies have focused on the association between these markers and prognosis in kinds of cancers. GEP–NETs are a type of relatively rare tumor, and the prognostic factors and surveillance strategy for GEP–NETs patients have not been well established due to the complexity and rarity of this disease. Therefore, a clear demonstration of the prognosis values of these easily applicable markers in GEP–NETs patients may help predict individual outcome and guide clinical decisions. In this study, we have summarized the published evidence on the association between the hematologic parameters and GEP–NETs outcomes. We found, in the field of this rare tumor, NLR, PLR, and platelet counts were all correlated with prognosis. Our pooled-analysis identified that NLR is an effective prognosis factor in GEP–NETs patients. The prognosis value of other hematologic parameters warrants interest and further study.
Most patients involved in the present study were PNET patients. The management of PNETs remains a big challenge because of their heterogeneous pathologic features and unpredictable clinical behaviors. Several markers have been identified as diagnosis markers or prognosis factors in PNETs. Chromogranin A (CgA) is the most commonly used biomarker and has been reported to be elevated in 50%–80% of PNET patients.Citation39 However, its prognosis value has been questioned due to some studies which showed there was no association between CgA and survival in PNETs.Citation40 Other potential markers include neurokinin A, pancreatic polypeptide, serotonin, neuron-specific enolase, etc., but their role is still unclear. Future research is needed to discover new markers and to determine which markers provide better prognostic information.Citation6,Citation41–Citation43 Although the number of studies investigating the predictive value of NLR in PNETs is much less than studies for other tumors, they all revealed that NLR was a remarkable prognosis marker for predicting both survival and recurrence in PNETs. Therefore, NLR has the potential to serve as a supplemental prognostic marker. Besides, NLR is an easily available marker obtained from routine blood tests, which enhances the practicality.
There are some limitations in the present study. Most of the included studies used ROC curve to determine a cut-off value for hematologic parameters.Citation27–Citation31 Therefore, the NLR and PLR were used as dichotomous variables. Additionally, since the cut-off values were artificially chosen, the clinicopathological characters between groups in each study were incomparable. Moreover, the hematologic parameters could be affected by different conditions and diseases, and the survival time of NETs is usually longer than other cancers because most NETs are biologically less aggressive, but all studies only used one result before treatment and did not monitor hematologic parameters during follow-up. Besides, because G3 tumors are invariably lethal, and the malignant potentials of G1 and G2 tumors were relative indolent, it is better for further studies to give additional information regarding the association between hematologic markers and prognosis in G1/G2 patients and G3 patients separately. We also noted that no study reported cancer-specific survival, which is a better outcome indicator than OS because of the relatively long survival time of patients with GEP–NETs. Last but importantly, due to the retrospective nature of the current study, patients may be prone to potential selection bias. Prospective and larger studies with a longer follow-up are required to confirm these findings.
In conclusion, this present systematic review and meta-analysis summarized the current evidence on the prognosis values of hematologic parameters in GEP–NETs. Our results showed that NLR was an effective prognostic predictor. The prognostic value of other hematologic parameters deserves further investigation. The conclusion should be limited mainly to PNETs due to the majority of included cases being PNETs. We recommend that future studies should use a continuous NLR variable and adopt a prospective and matched study design.
Acknowledgments
We gratefully acknowledge the National Natural Science Foundation of China (No. 81602172).
Disclosure
The authors report no conflicts of interest in this work.
References
- ModlinIMObergKChungDCGastroenteropancreatic neuroendocrine tumoursLancet Oncol200891617218177818
- FraenkelMKimMFaggianoAde HerderWWValkGDKnowledge NETworkIncidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literatureEndocr Relat Cancer2014213R153R16324322304
- TuragaKKKvolsLKRecent progress in the understanding, diagnosis, and treatment of gastroenteropancreatic neuroendocrine tumorsCA Cancer J Clin201161211313221388967
- OrgeraGKrokidisMCappucciMCurrent status of interventional radiology in the management of gastro-entero-pancreatic neuroendocrine tumours (GEP–NETs)Cardiovasc Intervent Radiol2015381132425366087
- CivesMSoaresHPStrosbergJWill clinical heterogeneity of neuroendocrine tumors impact their management in the future? Lessons from recent trialsCurr Opin Oncol201628435936627138571
- LandryCSCavanessKCelinskiSPreskittJBiochemical prognostic indicators for pancreatic neuroendocrine tumors and small bowel neuroendocrine tumorsGland Surg20143421521825493250
- KanakisGKaltsasGBiochemical markers for gastroenteropancreatic neuroendocrine tumours (GEP–NETs)Best Pract Res Clin Gastroenterol201226679180223582919
- GrivennikovSIGretenFRKarinMImmunity, inflammation, and cancerCell2010140688389920303878
- SethiGShanmugamMKRamachandranLKumarAPTergaonkarVMultifaceted link between cancer and inflammationBiosci Rep201232111521981137
- BaniyashMSade-FeldmanMKantermanJChronic inflammation and cancer: suppressing the suppressorsCancer Immunol Immunother2014631112023990173
- BuntSKClementsVKHansonEMSinhaPOstrand-RosenbergSInflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4J Leukocyte Biol2009856996100419261929
- GabrilovichDINagarajSMyeloid-derived suppressor cells as regulators of the immune systemNature Rev Immunol20099316217419197294
- CaddenIJohnstonBTTurnerGMcCanceDArdillJMcGintyAAn evaluation of cyclooxygenase-2 as a prognostic biomarker in mid-gut carcinoid tumoursNeuroendocrinology200786210411117700013
- KlöppelGClemensAThe biological relevance of gastric neuroendocrine tumorsYale J Biol Med199669169749041691
- Le Marc’hadourFBostFPeoc’hMRouxJJPasquierDPasquierBCarcinoid tumour complicating inflammatory bowel disease. A study of two cases with review of the literaturePathol Res Pract19941901211851192 discussion 1193–12007792207
- HaukimNBidwellJLSmithAJCytokine gene polymorphism in human disease: on-line databases, supplement 2Genes Immun20023631333012209358
- BerkovicMCJokicMMaroutJRadosevicSZjacic-RotkvicVKapitanovicSIL-6-174 C/G polymorphism in the gastroenteropancreatic neuroendocrine tumors (GEP–NETs)Experim Molec Pathol2007833474479
- BerkovicMCJokicMMaroutJRadosevicSZjacic-RotkvicVKapitanovicSIL-2 -330 T/G SNP and serum values-potential new tumor markers in neuroendocrine tumors of the gastrointestinal tract and pancreas (GEP–NETs)J Molec Med (Berlin)201088442342920049409
- ClarkeSJChuaWMooreMUse of inflammatory markers to guide cancer treatmentClin Pharmacol Therapeut2011903475478
- SchubertCHongSNatarajanLMillsPJDimsdaleJEThe association between fatigue and inflammatory marker levels in cancer patients: a quantitative reviewBrain Behav Immun200721441342717178209
- VermeireSVan AsscheGRutgeertsPThe role of C-reactive protein as an inflammatory marker in gastrointestinal diseasesNat Clin Pract Gastroenterol Hepatol200521258058616327837
- PorrataLFRistowKColganJPPeripheral blood lymphocyte/monocyte ratio at diagnosis and survival in classical Hodgkin’s lymphomaHaematologica201297226226921993683
- StotzMGergerAEisnerFIncreased neutrophil-lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancerBr J Cancer2013109241642123799847
- ChoHHurHWKimSWPre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatmentCancer Immunol Immunother2009581152318414853
- TaoSHaugUKuhnKBrennerHComparison and combination of blood-based inflammatory markers with faecal occult blood tests for non-invasive colorectal cancer screeningBr J Cancer201210681424143022454079
- TomitaMShimizuTAyabeTNakamuraKOnitsukaTElevated preoperative inflammatory markers based on neutrophil-to-lymphocyte ratio and C-reactive protein predict poor survival in resected non-small cell lung cancerAnticancer Res20123283535353822843942
- ArimaKOkabeHHashimotoDNeutrophil-to-lymphocyte ratio predicts metachronous liver metastasis of pancreatic neuroendocrine tumorsInt J Clin Oncol201722473473928285371
- LuoGLiuCChengHNeutrophil-lymphocyte ratio predicts survival in pancreatic neuroendocrine tumorsOncol Lett20171342454245828454419
- TongZLiuLZhengYPredictive value of preoperative peripheral blood neutrophil/lymphocyte ratio for lymph node metastasis in patients of resectable pancreatic neuroendocrine tumors: a nomogram-based studyWorld J Surg Oncol201715110828558772
- KaltenbornAMatzkeSKleineMPrediction of survival and tumor recurrence in patients undergoing surgery for pancreatic neuroendocrine neoplasmsJ Surg Oncol2016113219420226709239
- CaoLLLuJLinJXA novel predictive model based on preoperative blood neutrophil-to-lymphocyte ratio for survival prognosis in patients with gastric neuroendocrine neoplasmsOncotarget2016727420454205827275541
- SalmanTKazazSNVarolUPrognostic value of the pretreatment neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio for patients with neuroendocrine tumors: an Izmir Oncology Group StudyChemotherapy201661628128627070366
- YucelBBabacanNAKacanTSurvival analysis and prognostic factors for neuroendocrine tumors in TurkeyAsian Pacific J Cancer Prevent2014141166876692
- SakkaNSmithRAWhelanPA preoperative prognostic score for resected pancreatic and periampullary neuroendocrine tumoursPancreatology20099567067619684431
- SuLZhangMZhangWCaiCHongJPretreatment hematologic markers as prognostic factors in patients with nasopharyngeal carcinoma. A systematic review and meta-analysisMedicine20179611e636428296774
- GnjaticSBronteVBrunetLRIdentifying baseline immune-related biomarkers to predict clinical outcome of immunotherapyJ Immunother Cancer201754428515944
- ZhouXXuLHuangZThe hematologic markers as prognostic factors in patients with resectable gastric cancerCancer Biomark201617335936727434296
- AshrafganjoeiTMohamadianamiriMFarzanehFHosseiniMSArabMInvestigating preoperative hematologic markers for prediction of ovarian cancer surgical outcomeAsian Pacific J Cancer Prevent201617314451448
- KiddMBodeiLModlinIMChromogranin A: any relevance in neuroendocrine tumors?Curr Opin Endocrinol Diabetes Obesity20162312837
- ShermanSKMaxwellJEO’DorisioMSO’DorisioTMHoweJRPancreastatin predicts survival in neuroendocrine tumorsAnn Surg Oncol20142192971298024752611
- YaoJCPavelMPhanATChromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimusJ Clin Endocrinol Metabolism2011961237413749
- McCallCMShiCKleinAPSerotonin expression in pancreatic neuroendocrine tumors correlates with a trabecular histologic pattern and large duct involvementHuman Pathol20124381169117622221702
- ZandeeWTvan AdrichemRCKampKFeeldersRAvan VelthuysenMFde HerderWWIncidence and prognostic value of serotonin secretion in pancreatic neuroendocrine tumoursClin Endocrinol2017872165170
- TierneyJFStewartLAGhersiDBurdettSSydesMRPractical methods for incorporating summary time-to-event data into meta-analysisTrials200781617555582