Abstract
Purpose
Long noncoding RNAs (lncRNAs) are present in body fluids, but their potential as tumor biomarkers has never been investigated in malignant pleural effusion (MPE) caused by lung cancer. The aim of this study was to assess the clinical significance of lncRNAs in pleural effusion, which could potentially serve as diagnostic and predictive markers for lung cancer-associated MPE (LC-MPE).
Patients and methods
RNAs from pleural effusion were extracted in 217 cases of LC-MPE and 132 cases of benign pleural effusion (BPE). Thirty-one lung cancer-associated lncRNAs were measured using quantitative real-time polymerase chain reaction (qRT-PCR). The level of carcinoembryonic antigen (CEA) was also determined. The receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were established to evaluate the sensitivity and specificity of the identified lncRNAs and other biomarkers. The correlations between baseline pleural effusion lncRNAs expression and response to chemotherapy were also analyzed.
Results
Three lncRNAs (MALAT1, H19, and CUDR) were found to have potential as diagnostic markers in LC-MPE. The AUCs for MALAT1, H19, CUDR, and CEA were 0.891, 0.783, 0.824, and 0.826, respectively. Using a logistic model, the combination of MALAT1 and CEA (AUC, 0.924) provided higher sensitivity and accuracy in predicting LC-MPE than CEA (AUC, 0.826) alone. Moreover, baseline MALAT1 expression in pleural fluid was inversely correlated with chemotherapy response in patients with LC-MPE.
Conclusion
Pleural effusion lncRNAs were effective in differentiating LC-MPE from BPE. The combination of MALAT1 and CEA was more effective for LC-MPE diagnosis.
Introduction
Lung cancer remains the leading cause of cancer-related mortality globally, with almost one in five deaths attributable to it.Citation1 Non-small cell lung cancer (NSCLC) compromises >85% of all lung cancer cases.Citation2 Most patients with NSCLC are diagnosed with advanced disease, and malignant pleural effusion (MPE) presents in 11%–32% of these patients.Citation3 MPE can occur in patients with lung cancer of all histological types; however, it is particularly common in those with adenocarcinoma.Citation4 The importance of accurately assessing MPE was emphasized by the seventh edition of the tumor-node-metastasis classification for lung cancer, in which its status was reclassified as a stage IV disease.Citation5 Currently, the differential diagnosis between benign pleural effusion (BPE) and lung cancer-associated MPE (LC-MPE) is extremely difficult, and only 50%–60% of LC-MPE cases can be diagnosed by cytological examination.Citation6 Although thoracoscopic surgery and thoracotomy could improve the diagnostic power, they are too invasive for patients with a poor performance status, and many hospitals do not have these technologies, limiting their clinical application.Citation7 Carcinoembryonic antigen (CEA) in pleural effusion is the most commonly used biomarker for diagnosing LC-MPE, but the sensitivity of detection is often unsatisfactory.Citation8 Therefore, establishment of noninvasive biomarkers that could supplement CEA to improve the efficacy for diagnosing LC-MPE has important clinical implications.
Long noncoding RNAs (lncRNAs), which are commonly defined as transcripts longer than 200 nucleotides, play a critical role in the regulation of diverse cellular processes such as proliferation, cell cycle progression, cell growth and apoptosis, and cancer metastasis.Citation9 Recent studies have reported that lncRNAs, which are involved in tumorigenesis and tumor progression, are present in various biological fluids including plasma, saliva, and urine.Citation10–Citation13 Although the mechanisms by which lncRNAs are released into body fluids remain largely unknown, several studies have indicated that they can be secreted from tumor cells.Citation14 Thus, they could serve as biomarkers for cancer diagnosis. For example, Zhou et al reported that lncRNA H19 expression was significantly upregulated in the tumor tissue and plasma of gastric cancer patients and could be used to discriminate cancer patients from healthy controls.Citation15 In addition, Tang et al demonstrated that the lncRNAs RP11-160H22.5, XLOC_014172, and LOC149086 were upregulated in the tissue and plasma of hepatocellular carcinoma patients compared with their levels in cancer-free controls, and could act as biomarkers for predicting the occurrence and metastasis of hepatocellular carcinoma.Citation16 At present, although many lung cancer-associated lncRNAs have been found, their diagnostic or predictive value in pleural effusion has never been explored.
In the present study, 31 lncRNAs proven to have specificity for lung cancer in a previous study were chosen as candidate diagnostic markers. They were examined in pleural effusion, and their potential use as tumor markers for distinguishing LC-MPE from BPE was evaluated and compared with that of CEA in pleural effusion. The correlations between baseline lncRNAs expression in patients with LC-MPE and chemotherapy response were also investigated.
Materials and methods
Ethics statement
The study was approved by the Research Ethics Committee, Huai’an First People’s Hospital, Nanjing Medical University, and written informed consent was obtained from all patients.
Clinical samples
Three hundred and forty-nine patients with pleural effusion were recruited for this study from Huai’an First People’s Hospital, Nanjing Medical University between January 2013 and December 2015. The presence of pleural effusion was demonstrated by a chest computed tomography scan. All patients underwent thoracentesis and pleural fluid cytology. For patients with an exudate of unknown origin, a second thoracentesis, pleural biopsy, or thoracoscopy was performed.
We divided the enrolled patients into two groups: LC-MPE (n=217) and BPE (n=132). LC-MPE was diagnosed by the presence of malignant cells in pleural fluid or pleural biopsy specimens, or if patients had disseminated malignancy and there was no alternative explanation for pleural effusion. All of the lung cancer patients were newly diagnosed and showed no evidence of another malignant tumor. The primary pulmonary lesion was classified as 68 squamous cell carcinomas, and 149 adenocarcinomas. Pleural effusions were diagnosed as benign based on the clinical context and the absence of malignant cells in at least two separate samples from the same patients. BPE samples were obtained from patients with no clinical or radiological evidence of malignancy prior to the study. Of the 132 patients with BPE, 50 were caused by tuberculous pleurisy, 26 by congestive cardiac failure, and 56 by parapneumonic effusion. A detailed description of the patients can be found in the Supplementary materials.
Clinical data including age, gender, and CEA are presented in . The pleural effusion values of CEA were measured by electrochemiluminescence immunoassay using an analyzer (Hoffman-la-Roche Ltd, Basel, Switzerland).
Table 1 Clinical characteristics of patients
Pleural fluid was collected from each patient prior to any cancer-directed therapy. Briefly, the puncture site was chosen by ultrasound. The patient was positioned to sit on the edge of the bed, leaning forward, with his arms resting on a bedside table. After routine disinfection and draping, the epidermis was anesthetized with 2% lidocaine. Then, the needle was inserted into the pleural cavity. Once the pleural fluid was obtained, the needle was not advanced any further, and 10 mL pleural effusion was aspirated from each patient. All pleural samples were transported to the laboratory within 30 minutes of collection. The samples were then centrifuged at 12,000 g for 10 minutes at 4°C in a refrigerated microfuge. The supernatant from each sample was transferred to an RNase-free tube and stored at −80°C until total RNA extraction.
Of the 217 patients with LC-MPE, 162 cases received four cycles of cisplatin-based doublet chemotherapy, including cisplatin in combination with paclitaxel (n=37), cisplatin plus docetaxel (n=56), cisplatin plus pemetrexed (n=34), and cisplatin plus vinorelbine (n=35).
Clinical response evaluation
After the baseline evaluation, chemotherapy response was assessed after completion of the second and fourth cycle of chemotherapy. The response to treatment was assessed basically according to the Response Evaluation Criteria in Solid Tumor.Citation41
RNA extraction
Total RNA was isolated from pleural effusion samples using mirVana PARIS Kit (Ambion 1556; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s protocol. In brief, all pleural effusion samples were thawed on ice, and 400 μL of each sample was transferred to a tube containing an equal volume of 2× denaturing solution. Then, a volume of acid-phenol:chloroform was added equal to the total volume of the sample lysate plus the 2× denaturing solution. After centrifugation, the aqueous phase was recovered and transferred to a fresh tube. Subsequently, the lysate/ethanol mixture was passed through a filter cartridge, and the filter washed with wash solution. Finally, the RNA was eluted with 40 μL 95°C nuclease-free water, and stored at −80°C. Eluted RNA from each sample was quantified by NanoDrop ND-1000 (Thermo Fisher Scientific), and the mean amount of total RNA isolated from 400 μL of pleural effusion was 220.5 ng (range, 120.5–350.8 ng).
qRT-PCR analysis of lncRNAs expression
The concentrations of lncRNAs were quantified by quantitative real-time polymerase chain reaction (qRT-PCR) using SYBR® Premix Ex Taq™ II (Takara, Kyoto, Japan). The sequences of the qRT-PCR primers used in the present study are listed in . Briefly, a fixed volume of 5 μL of total RNA elute from 40 μL eluates was reverse transcribed in cDNA using PrimeScript™ RT reagent kit with gDNA Eraser (Takara; RR047A) according to the manufacturer’s instructions in a reaction volume of 20 μL. For the synthesis of cDNA, the reaction mixtures were incubated at 37°C for 15 minutes, followed by 85°C for 5 seconds, and then held at 4°C. Then, 2 μL of cDNA solution was amplified using 10 μL of SYBR® Premix Ex Taq™ (2×), 1.6 μL of primers, 0.4 μL of ROX Reference Dye II, and 6 μL of nuclease-free water in a final volume of 20 μL. All qRT-PCR procedures were run on the ABI 7500 Real-Time PCR machine (Thermo Fisher Scientific) under the following conditions: 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds and 60°C for 34 seconds. All amplification reactions were examined in triplicate and the specificity of each qRT-PCR reaction was confirmed by melt curve analyses. To normalize the expression levels of lncRNAs, we used GAPDH as the endogenous control. The expression levels of lncRNAs were calculated from the following equation: ∆Ct = mean CtlncRNAs − mean Ctreference; wherein lower ∆Ct values indicate higher expression. The relative expression of lncRNAs was calculated by using 2−∆∆CT method with GAPDH as internal control to normalize the data. Ct values >40 were considered negative.
Statistics analysis
The statistical significance of differences in lncRNA expression between groups was analyzed by Student’s t-test or the Mann–Whitney test. The Shapiro–Wilk test was used to determine whether lncRNA expression followed a normal distribution. The association between the level of lncRNA in pleural effusion and chemotherapy response was determined by the chi-square test. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were established to evaluate the sensitivity and specificity of the identified lncRNAs. The optimal cutoff value was calculated using the highest sum of sensitivity and specificity. Statistical analyses were performed using SPSS software (version 20.0; IBM Corporation, Armonk, NY, USA). All tests were two-sided and a p-value <0.05 was considered significant.
Results
Patient characteristics
Baseline characteristics of the study participants are shown in . In total, 132 effusions were diagnosed as benign and 217 were diagnosed as malignant. Of the 132 cases with BPE, there were 58 women (44%), and the median age was 57 years (range, 35–74 years). Of the 217 patients with LC-MPE, there were 93 women (43%), and the median age was 58 years (range, 32–78 years). As shown in , there were no significant differences in age or gender between the LC-MPE and BPE groups.
Of the 217 patients with LC-MPE, pleural fluid cytology was positive in 83 patients (38%) at the initial cytological analysis, and the remaining 134 patients were demonstrated by multiple thoracentesis (n=27), pleural biopsy (n=38), thoracoscopy (n=8), and the presence of disseminated malignancy; there were no alternative explanations for pleural effusion (n=61). Among the 217 patients, elevated levels of CEA (>5 ng/mL) were detected in 122 (56%) patients.
Lung cancer-associated lncRNAs are present in human pleural effusion samples
Thirty-one lncRNAs were selected based on published studies that reported their differential expression in lung cancer patients (). To investigate whether these lncRNAs were present in human pleural effusion, all 31 candidate lncRNAs were then detected by qRT-PCR in pleural effusion from 10 LC-MPE and 10 age- and sex-matched BPE.
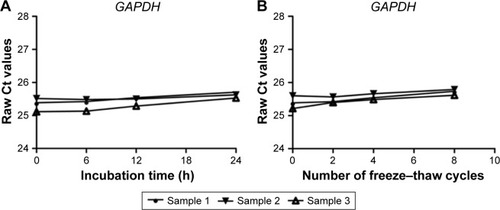
At present, there is no ideal housekeeping gene for the normalization of qRT-PCR data on pleural effusion lncRNAs. However, in our study, we found that the GAPDH level was stable in pleural effusion, and was not affected by age, sex, or pathology (, ). Thus, GAPDH was employed as an endogenous reference for the normalization of qRT-PCR data on lncRNAs in pleural effusion.
Table 2 Correlation between GAPDH level (raw Ct value) in human pleural effusion and clinicopathological factors of patients with BPE and LC-MPE
Figure 1 The stability of GAPDH in human pleural effusion.
We noticed that 11 (MAL1T1, UCA1, H19, AFAP1-AS1, SPRY4-IT1, PVT1, lncRNA-LET, PANDAR, CUDR, ANRIL, and NEAT-1) of the 31 analyzed lncRNAs were expressed at detectable levels in pleural effusion (the average Ct values were <34). Among them, three lncRNAs (lncRNA-LET, AFAP1-AS1, and UCA1) presented detection rates of <60% in both LC-MPE and BPE patients and were excluded from further analysis.
Validation of lung cancer-associated lncRNAs in human pleural effusion samples
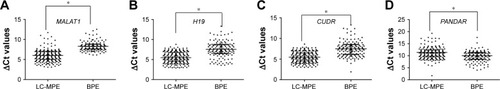
The expression levels of the remaining eight candidate lncRNAs were then measured by qRT-PCR in pleural effusions from all the subjects, including 217 LC-MPE and 132 BPE. As shown in , the levels of MALAT1, H19, and CUDR in pleural effusions were significantly higher in LC-MPE than in BPE, while the levels of PANDAR were significantly lower in LC-MPE than in the control group (). These results further suggest that lncRNAs can be utilized as a diagnostic tool for LC-MPE. However, the expression of SPRY4-IT1, ANRIL, NEAT-1, and PVT1 did not demonstrate any differences between LC-MPE and BPE (p>0.05). Therefore, we selected MALAT1, H19, CUDR, and PANDAR as candidates for further analysis.
Figure 2 Comparison of pleural fluid levels of MALAT1 (A), H19 (B), CUDR (C), and PANDAR (D) in LC-MPE and BPE.
Abbreviations: LC-MPE, lung cancer-associated malignant pleural effusion; BPE, benign pleural effusion; lncRNAs, long noncoding RNAs.
Determination of the stability of lung cancer-associated lncRNAs in human pleural effusion
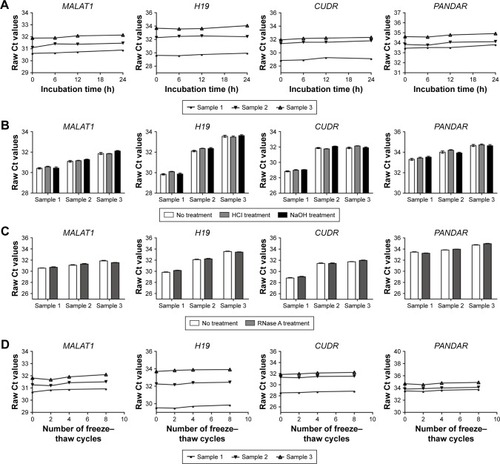
We next assessed the stability of the four lncRNAs in pleural effusion, given that this is an important foundation for utility as tumor markers. The pleural effusion samples were treated under harsh conditions including extended storage, strong acid and base treatment, freeze–thaw cycles, and RNase digestion. A total of three pleural effusion samples were used in this part of the study, and each pleural effusion sample was divided into four parts. As shown in , the incubation of a pleural effusion sample at room temperature for up to 24 hours or subjecting it to treatment with a low- or high-pH solution had minimal influence on the expression of MALAT1, H19, CUDR, and PANDAR, as detected by qRT-PCR. Moreover, the four lncRNAs remained stable when the pleural effusion samples were treated with RNase A digestion or multiple freeze–thaw cycles ().
Figure 3 The stability of lncRNAs in human pleural effusion.
Abbreviation: lncRNAs, long noncoding RNAs.
Diagnostic utility of MALAT1, H19, CUDR, PANDAR, and CEA for LC-MPE
The diagnostic performance of MALAT1, H19, CUDR, and PANDAR for distinguishing LC-MPE from BPE was evaluated by ROC curve analysis. As shown in , the ROC curves of MALAT1, H19, and CUDR exhibited strong separation between LC-MPE and BPE, with AUCs of 0.891 (95% CI: 0.854–0.928, p<0.001), 0.783 (95% CI: 0.730–0.836, p<0.001), and 0.824 (95% CI: 0.779–0.869, P<0.001), respectively, compared with CEA in pleural effusion with an AUC of 0.826 (95% CI: 0.784–0.869, p<0.001). The cutoff values of MALAT1, H19, and CUDR were 6.975, 6.595, and 6.005, respectively. The sensitivity, specificity, and accuracy of the three lncRNAs for distinguishing LC-MPE from BPE are listed in . Among all the candidates, MALAT1 showed the highest sensitivity and specificity in discriminating LC-MPE from BPE. However, PANDAR in pleural effusion could not clearly discriminate LC-MPE from BPE (; AUC, 0.657, p<0.001).
Table 3 Performance of pleural effusion lncRNAs and CEA in the differential diagnosis of LC-MPE from BPE
Figure 4 Evaluation of pleural fluid lncRNAs for the diagnosis of LC-MPE.
Abbreviations: LC-MPE, lung cancer-associated malignant pleural effusion; BPE, benign pleural effusion; lncRNAs, long noncoding RNAs; AUCs, area under the curves; CEA, carcinoembryonic antigen.
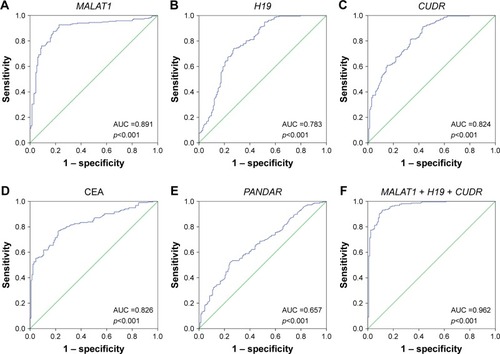
The use of the three lncRNAs together provided an even more powerful diagnostic tool for differentiating LC-MPE from BPE. Using a logistic model, the AUC for the three lncRNAs combined increased to 0.962 (95% CI: 0.942–0.982, p<0.001) with sensitivity of 91.7% and specificity of 89.4% ().
Combination of MALAT1 and CEA for LC-MPE diagnosis
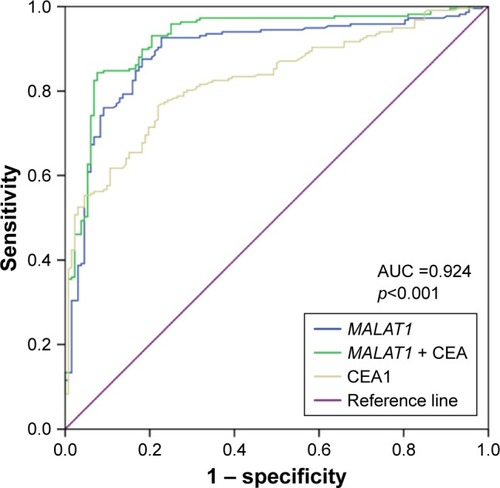
One of the main aims of this study was to increase the diagnostic sensitivity of CEA. The combination of MALAT1 and CEA for distinguishing LC-MPE from BPE was analyzed by logistic regression analysis. As shown in , this combination yielded an AUC of 0.924 (95% CI: 0.893–0.954, p<0.001), which was a significant improvement compared with those for CEA and MALAT1 alone. The sensitivity and specificity of this combination were 84.8% and 90.9%, respectively ().
Figure 5 ROC curves to compare the ability of CEA, MALAT1, and a combination of CEA and MALAT1, to discriminate LC-MPE from BPE.
Abbreviations: LC-MPE, lung cancer-associated malignant pleural effusion; BPE, benign pleural effusion; AUC, area under the curve; ROC, receiver operating characteristic; CEA, carcinoembryonic antigen.
Association of baseline lncRNA expressions in pleural effusion with chemotherapy response
The measurement of biomarkers is not only an effective means of detecting tumors, but also an important way of predicting treatment response. In this study, 162 lung cancer patients received cisplatin-based chemotherapy. After four cycles of chemotherapy, complete response (CR), partial response (PR), stable disease, and progressive disease were observed in five patients (3%), 52 patients (32%), 71 patients (44%), and 34 patients (21%), respectively. To investigate the association of baseline MALAT1 expression with chemotherapy response, the expression levels of MALAT1 in pleural effusion were categorized as high (n=81) and low (n=81) in relation to the median expression (∆Ct =6.155). The objective response rate (CR + PR) was significantly lower in patients with baseline MALAT1 <6.155 than in patients with MALAT1 ≥6.155 (22% vs 49%, p<0.001), demonstrating that MALAT1 in pleural fluid could be predictive of chemotherapy response in lung cancer before treatment. Unexpectedly, no significant correlations were observed between treatment response and clinicopathological parameters, such as age, gender, pathological type, and H19 and CUDR expressions in pleural effusion (p>0.05).
Discussion
To address several unanswered questions regarding the use of lncRNAs in pleural effusion biomarkers in LC-MPE, we focused on the pleural effusion levels of a set of 31 tumor-associated lncRNAs differently expressed between lung cancer tissues and adjacent normal tissues. The patient groups included patients diagnosed with LC-MPE and BPE. We observed that three lncRNAs, MALAT1, H19, and CUDR, could be clinically useful as biomarkers for LC-MPE diagnosis. Moreover, the combination of MALAT1 and CEA provided higher sensitivity and accuracy in predicting LC-MPE than CEA alone. In addition, our study also found that lung cancer patients with high relative expression levels of MALAT1 in the pleural fluid were correlated with chemotherapy resistance. To our knowledge, this is the first study wherein lncRNAs in pleural fluid have been shown to act as useful diagnostic and predictive biomarkers in LC-MPE.
To date, no other tumor markers have been identified as being suitable to replace CEA in pleural fluid for LC-MPE diagnosis. Elevated CEA in pleural fluid can predict LC-MPE with specificity of >90%, but the sensitivity is unsatisfactory.Citation4 Recently, many biomarkers such as CA15-3, CA-125, CA-199, CYFRA 21-1, surviving and LUNX mRNA were assessed for LC-MPE diagnosis, but the sensitivities of these antigens were remarkably low (ranging from 0.376 to 0.625).Citation17–Citation19 CEA in pleural fluid thus remains the most dependable of all biomarkers examined to date. Previous studies have indicated that lncRNAs are detectable in human fluids and revealed their potential utility as diagnostic markers in cancer. As early as 2002, de Kok et al demonstrated that lncRNA PCA3 can be examined in the urine of patients with prostate cancer.Citation20 The detection of lncRNA PCA3 in urine provided higher sensitivity and specificity for the diagnosis of prostate cancer than the widely used prostate-specific-agent test. Our study examined lung cancer-related lncRNAs in pleural effusions from patients with LC-MPE and BPE. We found that the lncRNAs MALAT1, CUDR, and H19 were significantly higher in LC-MPE than in BPE. MALAT1, also referred to as nuclear-enriched-abundant-transcript 2, was originally identified as a prognostic marker for metastasis and patient survival in gastric cancer and NSCLC.Citation21,Citation22 This lncRNA was widely expressed in normal human tissues, but was found to be overexpressed in multiple types of human malignancy, including hepatocellular carcinoma, breast, colon, lung, and prostate cancer, and it has served as a useful biomarker for the diagnosis of prostate and lung cancer.Citation23 According to our results, lncRNA MALAT1 in pleural effusion might be an effective tumor marker to discriminate LC-MPE from BPE, and the diagnostic performance was better than the use of CEA. The combination of MALAT1 and CEA improved the diagnostic ability (AUC, 0.924); in particular, the sensitivity increased to 84.8%.
qRT-PCR has become a versatile technique for examining gene expression.Citation24 The use of reference genes as internal controls is the most common approach for improving the comparability of gene expression data.Citation25 To produce reliable data on lncRNA expression levels, the selection of an appropriate reference gene is essential. A satisfactory reference gene should be present in all samples, and should also be stably expressed in all samples. Although several genes (β-actin, GAPDH, HPRT1, RPLP0) have been used as endogenous controls in the normalization of tissue lncRNA expression, no endogenous control for normalizing lncRNAs in pleural effusion has been established.Citation26 Our results demonstrated that GAPDH levels remained stable after prolonged incubation at room temperature or repeated freezing and thawing, and were not affected by age, gender, or pathology. This is in accordance with the work of Shao et al, who used GAPDH for the normalization of lncRNAs in gastric juice samples of gastric cancer patients and normal controls.Citation27
It has been reported that lncRNAs can be released into circulation from apoptotic and necrotic tumor cells and could be used to detect and monitor tumors.Citation28 The 31 lncRNAs chosen for the present study were carefully selected with the hope of proving that they were secreted from or leaked out of lung cancer cells. However, with this selection process, the majority of lncRNAs with altered expression in lung cancer tissues did not show the same alteration in levels in pleural fluid in our study, which strongly suggested that the tumor tissues or the malignant tumor cells were not the sole origin of lncRNAs in pleural fluid. Fourteen of the 31 analyzed lncRNAs which were highly expressed in lung cancer tissues were undetected in pleural fluid caused by lung cancer. At present, the precise mechanisms of lncRNAs released in pleural fluid are still unclear. There are several possible explanations for this: the first is that lncRNAs found in pleural effusion might be released from blood cells or normal tissues. For example, Pritchard et al demonstrated that blood cells were the major contributors to the circulating miRNAs and that perturbations in blood cell counts and hemolysis can alter plasma miRNA concentrations by up to 50-fold.Citation29 A second possible explanation is that lncRNAs found in pleural effusion are secreted and transferred from exosomes. Several studies have indicated that lncRNAs are abundant in exosomes and can be actively secreted from a variety of normal and tumor cells.Citation30,Citation31 Exosomes, which are automatically secreted, may transfer lncRNAs to body fluids.Citation32,Citation33 Another possible explanation is that the lncRNA levels in pleural fluid are correlated with immune responses. For example, some authors have speculated that the ncRNA signatures detected in patient plasma actually reflect the systemic responses to disease in the host microenvironment.Citation34 Clearly, further analysis should be carried out to clarify the source of lncRNAs in pleural fluid and to understand their exact function in tumor progression.
After diagnosis, chemotherapy or targeted therapies are the main methods of treating patients with MPE caused by lung cancer.Citation35,Citation36 At present, the most commonly used chemotherapeutic regimen is cisplatin-based combination chemotherapy, consisting of cisplatin with a third-generation chemotherapy agent such as docetaxel, pemetrexed, or gemcitabine.Citation37 However, NSCLC is poorly chemosensitive to most available agents with response rates ranging from 10% to 25%.Citation38 Thus, chemotherapy resistance is the main obstacle in the successful treatment of advanced NSCLC. In the present study, we found that baseline MALAT1 expression was inversely correlated with chemotherapy response in patients with LC-MPE. Several lncRNAs are known to be correlated with chemotherapy-sensitive phenotypes in cancers. We previously found that the lncRNA AFAP1-AS1 was significantly increased in the cisplatin-resistant KYSE30-R cell line compared with that in its parental cell line, and high expression of this lncRNA was significantly correlated with poor response to definitive chemoradiotherapy in patients with esophageal squamous cell carcinoma.Citation39 Liu et al also demonstrated that the lncRNA MEG3 was downregulated in lung cancer cells and partially regulated cisplatin resistance of tumor cells through the p53 signaling pathway.Citation40 To the best of our knowledge, the correlation between MALAT1 expression and resistance to chemotherapy for lung cancer has not been analyzed.
The present study provides the first clinical evidence that lncRNAs in pleural effusion might have diagnostic potential for differentiating LC-MPE from BPE. However, this study has some limitations. First, the total number of patients included is relatively small. Second, we confirmed that MALAT1 expression predicted resistance to cisplatin-based chemotherapy in patients with LC-MPE. However, the exact mechanisms involved in this remained unclear, and no survival analysis to assess the prognostic value of MALAT1 was performed. In addition, several unknown pathological factors potentially affecting the expression of lncRNAs in pleural effusion might have influenced our results.
Conclusion
The lncRNAs MALAT1, H19, and CUDR in pleural effusion are valuable biomarkers for the diagnosis of LC-MPE. In particular, MALAT1 in pleural effusion shows higher diagnostic sensitivity and accuracy for LC-MPE, meaning that it is effective when used in combination with CEA for discriminating between LC-MPE and BPE.
Author contributions
WWW, WGZ, and YST conceived and designed the experiments and were responsible for writing the manuscript. XLZ was responsible for data analysis. CHY recruited the patients and collected their clinical data. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
This work was supported by a grant from the National Natural Science Foundation of China (grant no H1617/81602118).
Supplementary materials
Tuberculous pleurisy was diagnosed if acid-fast bacillus was identified in pleural effusion, or caseous granuloma was found in pleural biopsy specimens, or a high concentration of pleural fluid adenosine deaminase was examined (>35 μ/L). Parapneumonic effusion was diagnosed when there was acute febrile illness with pulmonary infiltrate and responsiveness to antibiotic treatment in patients with pleural effusion. A diagnosis of congestive cardiac failure was made by findings of an enlarged heart, pulmonary venous congestion on the radiograph, and with response to congestive cardiac failure treatment.
Table S1 Primers sequences list
Disclosure
The authors report no conflicts of interest in this work.
References
- MinguetJSmithKHBramlagePTargeted therapies for treatment of non-small cell lung cancer – recent advances and future perspectivesInt J Cancer2016138112549256126537995
- RingnerMJonssonGStaafJPrognostic and chemotherapy predictive value of gene-expression phenotypes in primary lung adenocarcinomaClin Cancer Res201622121822926265693
- RyuJSRyuHJLeeSNPrognostic impact of minimal pleural effusion in non-small-cell lung cancerJ Clin Oncol201432996096724550423
- ShinYMYunJLeeOJDiagnostic value of circulating extracellular miR-134, miR-185, and miR-22 levels in lung adenocarcinoma-associated malignant pleural effusionCancer Res Treat201446217818524851110
- HanHSYunJLimSNDownregulation of cell-free miR-198 as a diagnostic biomarker for lung adenocarcinoma-associated malignant pleural effusionInt J Cancer2013133364565223354517
- BaoQLLiJSunWJiangHGZhuLRWangYDiagnostic utility of LUNX mRNA and VEGF mRNA in pleural fluid for differentiating benign from malignant originJpn J Clin Oncol201444121198120525425729
- TangYXuLSuperiority and clinical significance of Lunx mRNA in the diagnosis of malignant pleural effusion caused by pulmonary carcinomaJ Exp Clin Cancer Res2013323723759037
- LiangQLShiHZQinXJLiangXDJiangJYangHBDiagnostic accuracy of tumour markers for malignant pleural effusion: a meta-analysisThorax2008631354117573438
- QiuMXuYWangJA novel lncRNA, LUADT1, promotes lung adenocarcinoma proliferation via the epigenetic suppression of p27Cell Death Dis20156e185826291312
- WuYWangYQWengWWA serum-circulating long noncoding RNA signature can discriminate between patients with clear cell renal cell carcinoma and healthy controlsOncogenesis20165e19226878386
- XieZChenXLiJSalivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancerOncotarget2016718254082541927028998
- TongYSWangXWZhouXLIdentification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of ESCCMol Cancer201514325608466
- WangFRenSChenRDevelopment and prospective multicenter evaluation of the long noncoding RNA MALAT-1 as a diagnostic urinary biomarker for prostate cancerOncotarget2014522110911110225526029
- ReisEMVerjovski-AlmeidaSPerspectives of long non-coding RNAs in cancer diagnosticsFront Genet201233222408643
- ZhouXYinCDangYYeFZhangGIdentification of the long non-coding RNA H19 in plasma as a novel biomarker for diagnosis of gastric cancerSci Rep201551151626096073
- TangJJiangRDengLZhangXWangKSunBCirculation long non-coding RNAs act as biomarkers for predicting tumorigenesis and metastasis in hepatocellular carcinomaOncotarget2015664505451525714016
- NguyenAHMillerEJWichmanCSBerimIGAgrawalDKDiagnostic value of tumor antigens in malignant pleural effusion: a meta-analysisTransl Res2015166543243925953662
- ShitritDZingermanBShitritABShlomiDKramerMRDiagnostic value of CYFRA 21-1, CEA, CA 19-9, CA 15-3, and CA 125 assays in pleural effusions: analysis of 116 cases and review of the literatureOncologist200510750150716079317
- WuYKChenKTKuoYBHuangYSChanECQuantitative detection of survivin in malignant pleural effusion for the diagnosis and prognosis of lung cancerCancer Lett2009273233133518824294
- de KokJBVerhaeghGWRoelofsRWDD3(PCA3), a very sensitive and specific marker to detect prostate tumorsCancer Res20026292695269811980670
- GutschnerTHämmerleMEissmannMThe noncoding RNA MALAT1 is a critical regulator of the metastasis phenotype of lung cancer cellsCancer Res20137331180118923243023
- OkugawaYToiyamaYHurKMetastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasisCarcinogenesis201435122731273925280565
- QiPDuXThe long non-coding RNAs, a new cancer diagnostic and therapeutic gold mineMod Pathol201226215516522996375
- TaylorSWakemMDijkmanGAlsarrajMNguyenMA practical approach to RT-qPCR-Publishing data that conform to the MIQE guidelinesMethods2010504S1S520215014
- DavorenPAMcNeillRELoweryAJKerinMJMillerNIdentification of suitable endogenous control genes for microRNA gene expression analysis in human breast cancerBMC Mol Biol200897618718003
- WeberDGJohnenGCasjensSEvaluation of long noncoding RNA MALAT1 as a candidate blood-based biomarker for the diagnosis of non-small cell lung cancerBMC Res Notes2013651824313945
- ShaoYYeMJiangXGastric juice long noncoding RNA used as a tumor marker for screening gastric cancerCancer2014120213320332824986041
- QiPZhouXYDuXCirculating long non-coding RNAs in cancer: current status and future perspectivesMol Cancer20161513927189224
- PritchardCCKrohEWoodBBlood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studiesCancer Prev Res201153492497
- BerrondoCFlaxJKucherovVExpression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomesPLoS One2016111e014723626800519
- LiQShaoYZhangXPlasma long noncoding RNA protected by exosomes as a potential stable biomarker for gastric cancerTumour Biol20153632007201225391424
- MohankumarSPatelTExtracellular vesicle long noncoding RNA as potential biomarkers of liver cancerBrief Funct Genomics201615324925626634812
- KogureTYanIKLinWLPatelTExtracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancerGenes Cancer201347–826127224167654
- ChenGWangJCuiQCould circulating miRNAs contribute to cancer therapyTrends Mol Med2013192717323116601
- SuSLiTLuBThree-dimensional radiation therapy to the primary tumor with concurrent chemotherapy in patients with stage IV non-small cell lung cancer: results of a multicenter phase 2 study from PPRA-RTOG, ChinaInt J Radiat Oncol Biol Phys201593476977726530745
- SandlerAGrayRPerryMCPaclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancerN Engl J Med2006355242542255017167137
- YaoYYuanDLiuHGuXSongYPretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapyCancer Immunol Immunother201362347147922986452
- FortunatoOBoeriMMoroMMir-660 is downregulated in lung cancer patients and its replacement inhibits lung tumorigenesis by targeting MDM2–p53 interactionCell Death Dis20145e156425501825
- ZhouXLWangWWZhuWGHigh expression of long non-coding RNA AFAP1-AS1 predicts chemoradioresistance and poor prognosis in patients with esophageal squamous cell carcinoma treated with definitive chemoradiotherapyMol Carcinog201655122095210526756568
- LiuJWanLLuKThe long noncoding RNA MEG3 contributes to cisplatin resistance of human lung adenocarcinomaPLoS One2015105e011458625992654
- PengYLiZZhangSAssociation of DNA base excision repair genes (OGG1, APE1 and XRCC1) polymorphisms with outcome to platinum-based chemotherapy in advanced nonsmall-cell lung cancer patientsInt J Cancer2014135112687269624729390
