Abstract
Gastrointestinal stromal tumor (GIST) is an uncommon mesenchymal tumor, and has been shown to be associated with synchronous or metachronous second malignancies. Rare cases of coincident GIST and non-Hodgkin lymphomas (NHL) have been reported previously. Here, we report two cases of GIST and coincident primary breast lymphoma, an uncommon subtype of extranodal NHL. We propose that the exceedingly low likelihood of both these cancers occurring in these two patients by chance warrants examination for possible common oncogenic pathways in these lesions, possibly involving shared anti-apoptotic mechanisms. Further research is vital to elucidate common oncogenic pathways between such rare lesions.
Introduction
Gastrointestinal stromal tumor (GIST) is a rare mesenchymal tumor with an estimated incidence of 14.5 per million; ~5,000 new GIST cases are diagnosed annually in the USA.Citation1 Most cases of GIST are sporadic, with only 5% being attributable to genetic syndromes.Citation1
The diagnosis of synchronous or metachronous malignancies in patients with GIST has been reported to occur in 17%–33% of cases.Citation2–Citation4 The most common coincident malignancies are carcinomas of the prostate gland, kidney, and bladder.Citation2 Less commonly, non-Hodgkin lymphoma (NHL) has been shown to have significantly increased occurrence in patients with GIST, both before and after diagnosis of GIST.Citation2 The literature suggests that gastrointestinal (GI) tract extranodal NHL (ie, – mucosa-associated lymphoid tissue [MALT] lymphoma) is the most common NHL subtype observed in patients with coincident GIST and NHL.Citation5,Citation6
To our knowledge, no case report exists describing GIST with synchronous or metachronous primary breast lymphoma (PBL). PBL is an exceedingly rare subtype of extranodal NHL, with an incidence of approximately three per million in the USA.Citation7 Diffuse large B cell lymphoma (DLBCL) is the most common histology of PBL with extranodal marginal zone (MALT) lymphoma being the next most common.Citation7 The literature for synchronous cancers in PBL is much scarcer than that for GIST, with coincident invasive ductal carcinomas of the breast being the most common type of synchronous tumor with PBL.Citation6,Citation8 Here, we report two cases of coincident PBL and GIST. We discuss the pathogenesis of each neoplasm and the existing literature addressing possible common pathways, potentially through anti-apoptotic mechanisms, involved by both that could explain this clinical observation.
Case series
Case 1
A 76-year-old woman presented after an abnormality was detected in her left breast on screening mammography. Core biopsy revealed the lesion to be DLBCL, positive for Bcl-2 (diffuse), CD20, and CD45 positive. Subsequent fluorodeoxyglucose positron emission tomography (PET) and CT scans during staging evaluation for her PBL revealed a 5.0 × 6.0 cm mass involving the duodenum and the head of the pancreas (). Biopsy of the duodenal/pancreatic mass revealed GIST, staining positive for CD117 and CD34. The patient’s family history was notable for first-degree relatives with breast, lung, and colon cancers, and the patient’s social history was notable for remote tobacco usage (less than five pack-years) and chronic moderate alcohol consumption (two alcoholic servings daily).
Figure 1 Imaging for Case 1.
Abbreviations: DLBCL, diffuse large B cell lymphoma; FDG, fluorodeoxyglucose; PET, positron emission tomography; R-CHOP, rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone.
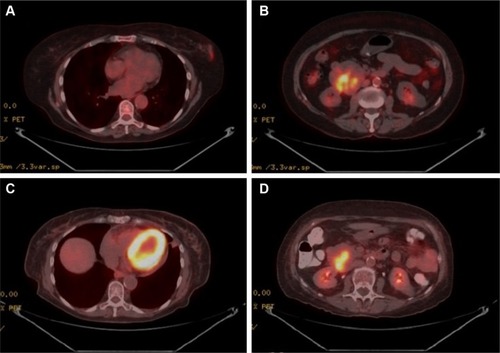
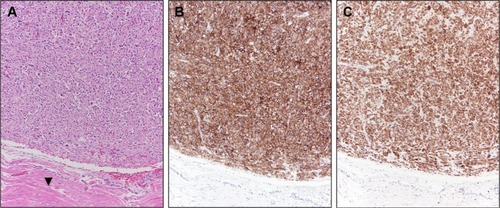
The patient underwent systemic therapy with six cycles R-CHOP (rituximab/cyclophosphamide/doxorubicin/vincristine/prednisolone) for DLBCL with concurrent imatinib mesylate for GIST. R-CHOP cycles were delivered every 21 days, with dosing as follows: rituximab intravenous (IV) 375 mg/mCitation2, cyclophosphamide IV 750 mg/mCitation2, doxorubicin IV 50 mg/mCitation2, and vincristine IV 1.4 mg/mCitation2 all on day 1, with prednisone per oral (PO) 100 mg/mCitation2 daily on days 1–5. Concurrent with R-CHOP cycles, 400 mg daily PO imatinib mesylate was given. No unexpected increased toxicities were observed with concurrent administration of R-CHOP and imatinib. After 10 months of imatinib (5 months after R-CHOP completion, continued at 400 mg daily PO dosing), the patient had repeat PET/CT performed to pre-operatively assess the duodenal GIST; this PET/CT revealed resolution of the breast mass, with interval decrease in size (4.6 × 5.2 cm) and central cavitation of the duodenal/pancreatic mass suggesting treatment-related necrosis, with continued metabolic activity suggesting persistent disease despite treatment response (). Patient, therefore, proceeded to pylorus-preserving pancreaticoduodenectomy, cholecystectomy, and retroperitoneal lymphadenectomy. Pathology revealed a 4.5 cm GIST invading the full thickness of duodenal wall, 1/18 lymph nodes involved by direct tumor extension, negative surgical margins, and less than five mitotic figures per 50 high-power field (HPF; ). No mutations in exons 9, 11, 13, or 17 of the c-KIT gene were reported. Immunohistochemical staining confirmed positivity of the GIST specimen for DOG1, a highly sensitive and specific marker for GIST,Citation9 as well as diffuse strong positive staining for Bcl-2 ().
Figure 2 Case 1 GIST surgical resection pathology findings.
Five months after pancreaticoduodenectomy, surveillance imaging revealed diffuse dissemination of disease. Tissue sampling was diagnostic of DLBCL with spread to both nodal and extranodal sites, including liver and bone. Owing to poor performance status, the patient pursued supportive care measures alone and passed away 3 months after relapse of lymphoma and 20 months after initial diagnosis of PBL.
Case 2
A 52-year-old woman presented with a screening-mammography-detected abnormality, diagnosed as an extra-nodal marginal zone (MALT) lymphoma of the right breast after excisional biopsy. This histologic diagnosis prompted definitive radiotherapy (RT) to the involved whole breast to 40 Gy in 20 daily fractions (representing the standard of care at the time; RT doses in this setting have since decreased). The patient’s family history was notable for prostatic and lung adenocarcinomas in first-degree relatives, and her social history was negative for tobacco or alcohol usage.
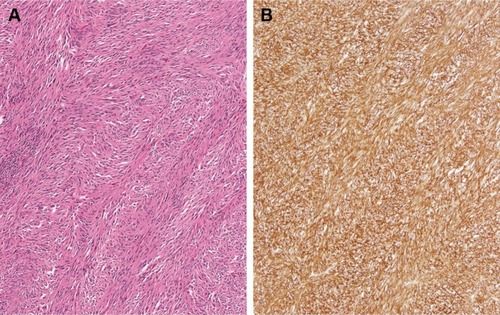
Twelve months after completion of RT for her breast MALT lymphoma, follow-up surveillance PET/CT imaging revealed a new hypermetabolic large abdominopelvic mass as well as a new right orbital mass. Biopsy of the right orbital mass revealed recurrent extranodal marginal zone lymphoma, and for this she was treated with rituximab monotherapy for 9 months; this resulted in a metabolic complete response in the orbit and the patient has remained disease-free from her marginal zone lymphoma since that time (8 years ago) until her most recent follow-up. While the patient was receiving rituximab for her recurrent lymphoma, she underwent abdominopelvic mass excision which revealed a GIST of the small intestinal serosa measuring 21×18 cm with four mitotic figures per 50 HPF, negative margins, and positive tumor staining CD117 and CD34 (). She received adjuvant imatinib for 12 months. She went on to have four local (abdominopelvic) recurrences of her GIST, all managed with a combination of surgical resection and (neo)adjuvant imatinib over the subsequent 7 years to the time of last follow-up. Pathology from subsequent resections revealed exon 11 c-KIT mutation. At the time of last follow-up, the patient was alive with recurrent disease (representing her fifth recurrence), with plans for neoadjuvant sorafenib and surgical resection.
Figure 3 Case 2 GIST surgical resection pathology findings.
Abbreviation: GIST, gastrointestinal stromal tumor.
Discussion
Here, we report on two cases of coincident PBL and GIST. From a statistical perspective, it is noteworthy that the chance of these entities occurring together randomly iŝ4.5 per 100 billion if no interaction is assumed between these diagnoses. However, given the known association of GIST with NHL, it is more likely that as-of-yet unknown factors are driving the coincidence of GIST with PBL in these patients.Citation10
According to one institutional review, patients with GIST and a second malignancy do not have significantly decreased survival.Citation3 Moreover, the median OS for non-metastatic GIST in the imatinib era was 13.7 years in a study examining data from the Life Raft Group Registry, which parallels the prolonged survival despite numerous GIST relapses in the second case.Citation11 These observations suggest that treatment strategies in cases of malignancies coinciding with GIST should often focus on the non-GIST malignancy.
Primary breast DLBCL represents a high-risk subtype of DLBCL, carrying substantial risk of central nervous system relapse as well as contralateral breast relapse.Citation12,Citation13 Data, including series from our institution, have demonstrated high rates of Bcl-2 overexpression in primary breast DLBCL, consistent with the patient from Case 1.Citation14,Citation15 Similarly, primary breast MALT lymphomas appear to carry higher risk of relapse, particularly distant relapse, compared with other primary MALT lymphomas, including those of the GI tract.Citation16,Citation17 Our data have shown overexpression of Bcl-10 in the vast majority of primary breast MALT lymphomas.Citation15 Unfortunately, breast lymphoma tissue from the patient in Case 2 was not available for Bcl-10 testing, but institutional reports from other primary breast MALT lymphoma patients highlights characteristic Bcl-10 overexpression in these rare tumors.Citation15 Both Bcl-2 and Bcl-10 appear to play critical roles in the pathogenesis of these respective lymphomas through anti-apoptotic activity. Bcl-2 in DLBCL has been shown to prevent chemotherapy-related apoptosis and, therefore, has been shown to carry prognostic significance.Citation18,Citation19 Bcl-10 in MALT lymphomas has similarly been shown to interact with the MALT1 gene product, resulting in inhibition of apoptosis.Citation20 In both, primary breast DLBCL and MALT lymphoma, anti-apoptotic pathways appear to be central components of oncogenesis.
GISTs, mesenchymal tumors believed to arise from the interstitial cells of Cajal (part of the autonomic nervous system), are characterized by c-KIT and PDGFRα mutations.Citation21,Citation22 While most GIST lesions have been shown to have c-KIT or PDGFRα mutations, ~10% of these tumors are wild-type for both (the patient in Case 1 [], whose GIST was negative for c-KIT mutations, though PDGFRα mutational testing was unavailable).Citation23,Citation24 It is noteworthy that exon 11 c-KIT mutant GIST patients have been recently shown to benefit from prolonged adjuvant imatinib for 3 years as opposed to 1 year.Citation25 Consistent with these data, the patient in Case 2 experienced first relapse after only 1 year of adjuvant imatinib.
Looking toward common molecular oncogenic mechanisms between PBL and GIST, it has been shown that Bcl-2 is upregulated in GIST tissue as compared with normal gastric tissue.Citation26 Survival advantages in PBLs through anti-apoptotic activity of Bcl-2/Bcl-10, discussed previously, appear to potentially occur in GIST lesions as well. While Bcl-2 expression analysis was not available for GIST specimen for the patient in Case 2, the strongly positive Bcl-2 expression for the GIST specimen in Case 1 () supports concurrent overexpression of Bcl-2 in both this patient’s GIST as well as her primary breast DLBCL. Common pathways promoting overexpression of anti-apoptotic proteins, such as Bcl-2, remain opaque. Preliminary data suggest that miR-21 may regulate Bcl-2 expression in both NHL and GIST cell lines, offering one hypothetical shared mechanism for upregulation of anti-apoptotic pathways across these different tumors.Citation26–Citation29 It is our hope that such speculation on common molecular pathways between these rare tumors spurs research into the underlying oncogenic pathways shared by these lesions, which may reveal as-of-yet-undiscovered oncogenic mechanisms in each of these tumors. With that in mind, the absence of c-KIT mutations in the GIST for Case 1 is noted, suggesting alternative molecular pathways that have not yet been characterized.
Conclusion
We report two patients with coinicident GIST and PBL. Given the improbability of stochastic co-occurrence of such uncommon tumors, there may a common underlying aberration (or aberrations) driving both lesions. In describing this association, we speculate that common anti-apoptotic pathways may be shared by these malignancies, and further research is needed to elucidate shared oncogenic mechanisms between GIST and PBL.
Consent
Written informed consent was obtained from the patients/next of kin for this publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- NilssonBBümmingPMeis-KindblomJMGastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era – a population-based study in western SwedenCancer2005103482182915648083
- MurphyJDMaGLBaumgartnerJMIncreased risk of additional cancers among patients with gastrointestinal stromal tumors: a population-based studyCancer2015121172960296725930983
- PandurenganRKDumontAGAraujoDMSurvival of patients with multiple primary malignancies: a study of 783 patients with gastrointestinal stromal tumorAnn Oncol201021102107211120348145
- AgaimyAWünschPHSobinLHLasotaJMiettinenMOccurrence of other malignancies in patients with gastrointestinal stromal tumorsSemin Diagn Pathol200623212012917193825
- SalarARamónJMBarrancoCDouble diagnosis in cancer patients and cutaneous reaction related to gemcitabine: Case 1. Synchronous mucosa-associated lymphoid tissue lymphoma and gastrointestinal stromal tumors of the stomachJ Clin Oncol200523287221722316192606
- KaffesAHughesLHollinsheadJKatelarisPSynchronous primary adenocarcinoma, mucosa-associated lymphoid tissue lymphoma and a stromal tumor in a Helicobacter pylori-infected stomachJ Gastroenterol Hepatol20021791033103612167128
- ThomasALinkBKAltekruseSRomittiPASchroederMCPrimary breast lymphoma in the United States: 1975–2013J Natl Cancer Inst20171096
- SiddiquiFAMaheshwariVAlamKJainACoexistent non-Hodgkins lymphoma and ductal carcinoma breast: diagnosis on fine needle aspiration cytologyDiagn Cytopathol2011391076776921919216
- HwangDGQianXHornickJLDOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocksAm J Clin Pathol2011135344845321350101
- KaranikasMMachairiotisNZarogoulidisPNon-Hodgkin lymphoma and GIST: molecular pathways and clinical expressionsOnco Targets Ther2012543343823251094
- CallJWalentasCDEickhoffJCScherzerNSurvival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registryBMC Cancer2012129022429770
- AvivATadmorTPolliackAPrimary diffuse large B-cell lymphoma of the breast: looking at pathogenesis, clinical issues and therapeutic optionsAnn Oncol20132492236224423712546
- HoseinPJMaraguliaJCSalzbergMPA multicentre study of primary breast diffuse large B-cell lymphoma in the rituximab eraBr J Haematol2014165335836324467658
- YoshidaSNakamuraNSasakiYPrimary breast diffuse large B-cell lymphoma shows a non-germinal center B-cell phenotypeMod Pathol200518339840515492762
- TalwalkarSSValbuenaJRAbruzzoLVMALT1 gene rearrangements and NF-kappaB activation involving p65 and p50 are absent or rare in primary MALT lymphomas of the breastMod Pathol200619111402140816917511
- MartinelliGRyanGSeymourJFPrimary follicular and marginal-zone lymphoma of the breast: clinical features, prognostic factors and outcome: a study by the International Extranodal Lymphoma Study GroupAnn Oncol200920121993199919570964
- TeckieSQiSLovieSLong-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intentInt J Radiat Oncol Biol Phys201592113013725863760
- GascoyneRDAdomatSAKrajewskiSPrognostic significance of Bcl-2 protein expression and Bcl-2 gene rearrangement in diffuse aggressive non-Hodgkin’s lymphomaBlood19979012442519207459
- MiyashitaTReedJCBcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell lineBlood19938111511578417786
- VegaFMedeirosLJMarginal-zone B-cell lymphoma of extranodal mucosa-associated lymphoid tissue type: molecular genetics provides new insights into pathogenesisAdv Anat Pathol20018631332611707622
- KindblomLGRemottiHEAldenborgFMeis-KindblomJMGastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of CajalAm J Pathol19981525125912699588894
- CorlessCLBarnettCMHeinrichMCGastrointestinal stromal tumours: origin and molecular oncologyNat Rev Cancer2011111286587822089421
- BaiCGHouXWWangFStem cell factor-mediated wild-type KIT receptor activation is critical for gastrointestinal stromal tumor cell growthWorld J Gastroenterol201218232929293722736916
- LambaGAmbraleSLeeBGuptaRRafiyathSMLiuDRecent advances and novel agents for gastrointestinal stromal tumor (GIST)J Hematol Oncol201252122569033
- JoensuuHWardelmannESihtoHEffect of KIT and PDGFRA mutations on survival in patients with gastrointestinal stromal tumors treated with adjuvant imatinib: an exploratory analysis of a randomized clinical trialJAMA Oncol20173560260928334365
- CaoCLNiuHJKangSPCongCLKangSRmiRNA-21 sensitizes gastrointestinal stromal tumors (GISTs) cells to imatinib via targeting B-cell lymphoma 2 (Bcl-2)Eur Rev Med Pharmacol Sci201620173574358127649657
- GoHJangJYKimPJMicroRNA-21 plays an oncogenic role by targeting FOXO1 and activating the PI3K/AKT pathway in diffuse large B-cell lymphomaOncotarget2015617150351504925909227
- LiuKduJRuanLMicroRNA-21 regulates the viability and apoptosis of diffuse large B-cell lymphoma cells by upregulating B cell lymphoma-2Exp Ther Med20171454489449629067124
- BaiHWeiJDengCYangXWangCXuRMicroRNA-21 regulates the sensitivity of diffuse large B-cell lymphoma cells to the CHOP chemotherapy regimenInt J Hematol201397222323123275230
