Abstract
Introduction
Radiofrequency ablation (RFA) is the foremost treatment option for advanced hepatocellular carcinoma (HCC), however, rapid and aggressive recurrence of HCC often occurs after RFA due to epithelial–mesenchymal transition process. Although combination of RFA with sorafenib, a molecular targeted agent, could attenuate the recurrence of HCC, application of this molecular targeted agent poses a heavy medical burden and oral administration of sorafenib also brings severe side effects.
Materials and methods
In this study, we prepared an apatinib microcrystal formulation (Apa-MS) that sustainably releases apatinib, a novel molecular targeted agent, for advanced HCC treatment. We injected apatinib solution or Apa-MS into subcutaneous HCC tumors.
Results
It was found that Apa-MS exhibited slow apatinib release in vivo and in turn inhibited the epithelial–mesenchymal transition of HCC cells for extended time. Moreover, in rodent HCC model, Apa-MS enhanced the antitumor effect of RFA treatment.
Conclusion
Based on these results, we conclude that Apa-MS, a slow releasing system of apatinib, allows apatinib to remain effective in tumor tissues for a long time and could enhance the antitumor effect of RFA on HCC.
Introduction
HCC poses a heavy medical burden worldwide, especially in the People’s Republic of China, due to the high infection rate of hepatitis viruses (hepatitis B or C virus).Citation1,Citation2 Patients often suffer from advanced HCC due to lack of early diagnostic techniques.Citation3,Citation4 RFA can selectively destroy HCC tissues, and hence, it is believed to be a suitable treatment option for advanced HCC patients with cirrhosis and compromised liver function.Citation5–Citation7 However, rapid/aggressive recurrence of HCC after RFA treatment is a major drawback of this approach.Citation8,Citation9 It has been reported that the EMT induced by RFA would be a major reason of HCC recurrence post-RFA, and combination of RFA and molecular targeted agent sorafenib could help to inhibit this process.Citation10,Citation11 However, there are some obstacles for sorafenib application: 1) advanced HCC patients often have compromised gastrointestinal digestive function that attenuates the absorption of orally administered sorafenib;Citation12–Citation14 2) oral administration of sorafenib will result in systemic distribution, making local drug concentration insufficient;Citation15 and 3) the high daily dose (over 800 mg) imposes a heavy financial burden.Citation15 Therefore, it is valuable to develop novel and more effective strategies to enhance the antitumor effect of RFA on HCC.
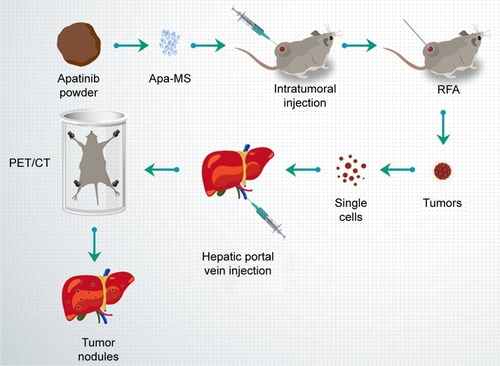
Apatinib is a newly approved molecular targeting drug for the treatment of advanced HCC.Citation16,Citation17 However, apatinib is insoluble in water, and the daily dose of apatinib-mesylate tablets is very high (over 850 mg).Citation18,Citation19 Microcrystallization is a pharmaceutical process that converts drug powder into microcrystals with a diameter of 30–50 μm.Citation20 Microcrystals have been used to improve the absorption of orally administered drugs due to the fact that microcrystals can fully contact and mix with digestive fluid.Citation20 Here, for the first time, we report the preparation of a microcrystal formulation that extends the release time of apatinib in vivo. By preparing Apa-MS and studying its antitumor effect in tumor models, we found apatinib was slowly released from Apa-MS in vivo. This approach could overcome the limitation of solubility of apatinib and condense drug concentration in tumor without affecting the surrounding tissue. We also examined whether single-dose administration of Apa-MS could achieve accurate apatinib delivery and enhance the antitumor effect of RFA on HCC cells. presents the workflow of the study.
Figure 1 Workflow of the study. Apatinib powder was formulated to Apa-MS and injected into HCC tumors, and then tumors were treated by RFA. After 2–4 weeks, tumors were harvested, and single cells were separated and injected into liver via hepatic portal vein. The nodules in liver formed by HCC cells were measured by PET/CT screening.
Materials and methods
Cell line and agents
MHCC97-H cells (a highly aggressive HCC cell line) was purchased from the Type Culture Collection of the Chinese Academy of Sciences and maintained under conditions described in our previous work.Citation21,Citation22 Apatinib was a gift from Jiangsu Hengrui Medicine Co., Ltd., Lianyungang, People’s Republic of China. Sodium dodecyl sulfate, dimethyl sulfoxide, polyethylene glycol 400 and Tween 80 were purchased from Sigma-Aldrich Corporation, St Louis, MO, USA.
Preparation of apatinib formulations
For preparing Apa-Sol, apatinib was dissolved in DMSO, PEG400 and Tween 80. Then, the solution was carefully and slowly diluted with physiological saline, accompanied by ultrasonic treatment or churning. The ultimate concentrations of DMSO, PEG400 and Tween 80 in solution were 1%, 4% and 4%, respectively, and the concentration of apatinib was almost 1 mg/mL. For preparing apatinib microcrystals,Citation23 1.5 g apatinib was dispersed into 50 mL aqueous solution with 6.25% Tween 80 and mixed by magnetic stirring to obtain a relatively uniform coarse suspension. The microcrystals were prepared using a MiniZeta (NETZSCH Machinery and Instruments Co., Ltd., Burlington, MA, USA) machine equipped with the grinding media of yttrium-stabilized zirconium oxide beads (0.6 mm in diameter). The coarse suspension was transferred to the milling bowl, and the agitator speed was set at 3,000 rpm. The apatinib concentration in Apa-MS was almost 30 mg/mL.
Subcutaneous tumor
All the animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the 302nd Hospital of People’s Liberation Army, Beijing, People’s Republic of China. Nude SCID mice aged 4–6 weeks were purchased from Si-Bei-Fu Biotechnology Corporation, Beijing, People’s Republic of China. All animal studies were performed in accordance with the UK Animals (Scientific Procedures) Act 1986 and associated guidelines. To produce a subcutaneous tumor model,Citation24 MHCC97-H cells were injected into nude SCID mice (1 × 106 cells per animal). After 2–3 weeks, the tumoral volume reached almost 1,200 mm3.
Pharmacokinetic experiments of apatinib formulations
For in vitro release experiments, the release behavior of Apa-MS was identified by vortex shock method. Apa-MS was added to 10 mL physiological saline with 0.1% Tween 80 and 0.9% NaCl. At different time points, 1 mL solution was sucked out for analysis, and the volume was made up to 10 mL. The release of apatinib into physiological saline at each time point was identified. For in vivo release experiments, 50 μL Apa-Sol or Apa-MS was intratumorally injected into subcutaneous tumor formed by MHCC97-H cells. At indicated time points, tumor tissues were harvested, and apatinib was extracted using acetonitrile. The amount of apatinib was identified by liquid chromatography–tandem mass spectrometry following the methods described by Allard et alCitation25 and He et al.Citation26
RFA treatment
MHCC97-H cells were injected into nude mice (1 × 106 cells per animal). After 4–6 weeks, the tumoral volume reached almost 1,200 mm3. RFA of the subcutaneous tumors was performed using a thyroid ablation needle (cat. no UniBlate 700-103587 17G; RITA Company, Crystal Lake, IL, USA). The ablation time was 3–5 minutes, and the temperature was 65°C–70°C. After 2–3 weeks of RFA treatment, the tumoral volume was calculated using the following formula: widthCitation2 × length/2. Tumors were harvested 16 days after RFA treatment, and their weights were measured.
qPCR experiments
Total RNA of cells or clinical specimens was extracted using a PARIS™ kit (Applied Biosystems; Thermo Fisher Scientific Corporation, Waltham, MA, USA) and reverse transcribed by MultiScribe™ Reverse Transcriptase (Applied Biosystems; Thermo Fisher Scientific Corporation). The qPCR experiments were performed as described previously.Citation27 The level of β-actin mRNA was measured as an internal control. Primers used in qPCR are listed in .
Table 1 Primers used in this work
Intrahepatic tumor model
To produce an in situ liver tumor model,Citation28,Citation29 HCC cells were separated from subcutaneous tumors formed by MHCC97-H cells and directly inoculated into the right lobe of the liver of mice (1 × 105 cells per animal). After 4–8 weeks, nude mice were injected intravenously with 100 μCi of 18F-FDG, and the animals were examined using a PET/CT scanner (Philips Corp., Amsterdam, the Netherlands). Two-minute CT and 10-minute PET scans were performed 45 minutes after the 18F-FDG injection. A NaI (Tl) well counter (China Atom Corp., Beijing, People’s Republic of China) was used to measure the radioactivity of organs (liver) and blood.Citation29,Citation30 The liver organ photographs were analyzed using ImageJ Software (version 1.51j8; National Institutes of Health, Bethesda, MD, USA). The percentage of red areas indicated the relative HCC amount.Citation29,Citation30
Statistical analysis
All statistical analyses were performed using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA). Statistical significance was analyzed by two-way ANOVA with Bonferroni correction. Differences between groups were examined by paired-sample t-test.
Results
Characterization of Apa-MS
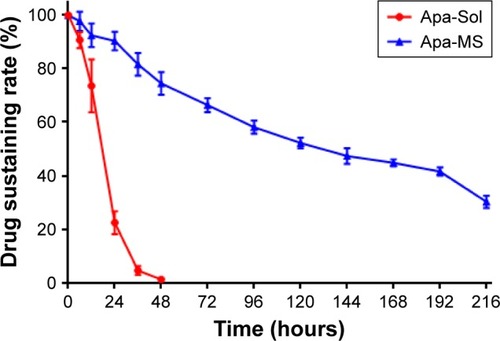
Apa-MS was prepared as described in the Materials and methods section. As shown in , Apa-MS contained irregularly shaped crystals with a particle diameter of 30–50 μm. Apa-MS released apatinib at a slow speed, and almost 60% (40.24%±3.52%) of apatinib remained after 9 days in vitro (). Next, the in vivo release of apatinib was examined. We prepared Apa-Sol as a control. Apa-Sol or Apa-MS was intratumorally injected into subcutaneous MHCC97-H tumors. Tumor tissues were harvested at indicated time points, and apatinib concentration was tested. As shown in , following single-dose administration of Apa-Sol, apatinib was completely cleared from tumor tissues at 48 hours, whereas clearance of Apa-MS was much slower: almost 30% (30.2%±2.00%) of Apa-MS remained in tumors at 216 hours (9 days).
Figure 2 Particle size and in vitro release rate of Apa-MS. (A) Apa-MS has an average particle size of 30–50 μm. Arrow indicates Apa-MS. Scale bar = 50 μm. (B) The in vitro release curve of apatinib was plotted with drug release (%) at indicated time points.
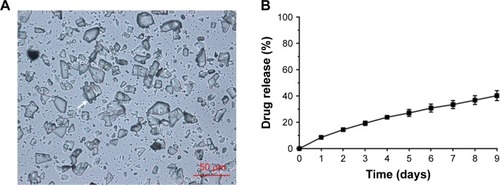
Figure 3 The in vivo release curve of apatinib formulations. Apa-Sol or Apa-MS was injected in tumor tissues. The release rates of apatinib in tumors from Apa-Sol or Apa-MS at the indicated time points are shown (mean ± SD, n = 10).
Apa-MS enhanced RFA-induced inhibition of the growth of HCC cells in vivo
Apa-Sol or Apa-MS was injected intratumorally into RFA-treated subcutaneous HCC tumor model. As shown in , Apa-MS administration reduced the tumor growth and enhanced the effect of RFA treatment. It has been previously reported that RFA induced the EMT of HCC cells by decreasing the expression of E-cadherin and increasing the expression of N-cadherin and vimentin.Citation31 Therefore, we examined the transcriptional level of these proteins in tumor tissues after the subcutaneous HCC tumor growth experiments. As shown in , an increase in the mRNA expression of E-cadherin and decrease in the mRNA expression of N-cadherin and vimentin were observed. Apa-MS also attenuated the EMT process of HCC cells induced by RFA at mRNA level. Moreover, the protein levels of E-cadherin, N-cadherin and vimentin were examined (). Single-dose administration of Apa-MS was found to inhibit the RFA-induced EMT at protein level (). Thus, we found that single-dose administration of Apa-MS inhibited the RFA-induced EMT in HCC.
Figure 4 Single dose of Apa-MS but not Apa-Sol inhibited the in vivo growth of HCC cells and enhanced the effect of RFA on subcutaneous HCC model. The experimental animals were divided into the following groups: no-treatment group (negative control), solvent control group, Apa-Sol group, Apa-MS group, RFA group, RFA + solvent control group, RFA + Apa-Sol group and RFA + Apa-MS group. Results are shown as (A) photographs, (B) volume (mean ± SD, n = 8) and (C) weight of tumors (mean ± SD, n = 8). *P<0.05.
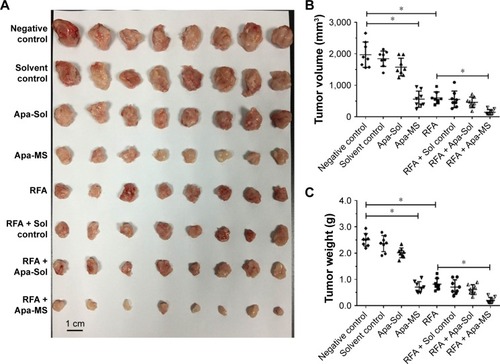
Figure 5 Apa-MS but Apa-Sol inhibited the EMT of HCC cells induced by RFA at mRNA level. Tumors from solvent control group, RFA group, Apa-Sol group, Apa-MS group, RFA + Apa-Sol group and RFA + Apa-MS group were harvested, and total mRNA was extracted. The relative mRNA level of (A) E-cadherin, (B) N-cadherin and (C) vimentin is shown as mean ± SD. *P<0.05.
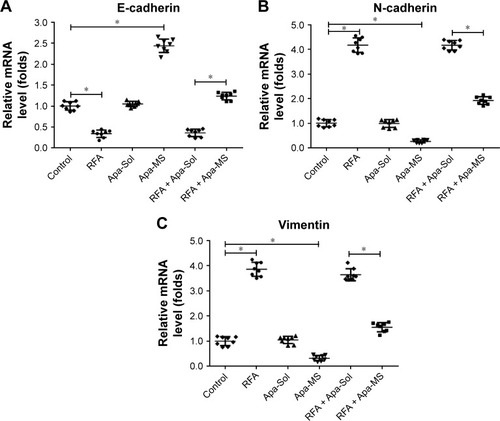
Figure 6 Apa-MS but Apa-Sol inhibited the EMT of HCC cells induced by RFA at protein level. Tumors from solvent control group, RFA group, Apa-Sol group, Apa-MS group, RFA + Apa-Sol group or RFA + Apa-MS group were harvested, and total protein was extracted. The protein level of E-cadherin, N-cadherin and vimentin was examined by their antibodies. GAPDH was chosen as a loading control.
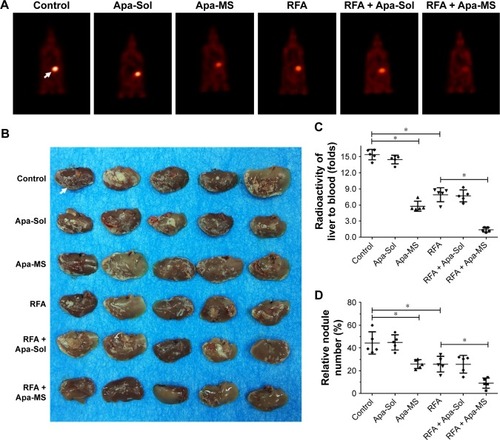
We also tested the effect of Apa-MS on intrahepatic HCC model, using PET/CT imaging of whole animals and liver organ photographs. As shown in , PET/CT images of whole animals indicated that RFA treatment decreased the intrahepatic growth of HCC cells and Apa-MS enhanced the effect of RFA. Moreover, HCC cells formed multifocal nodules in liver. RFA treatment decreased the nodules formation of HCC cells, and Apa-MS enhanced the antitumor effect of RFA (). Quantitative results including radioactivity ratio of liver to blood () and relative nodule number (percentage of nodules in liver) () confirmed the findings of PET images of whole animal () and liver organ images (). These in vivo data support our hypothesis that Apa-MS enhances the effect of RFA treatment on HCC.
Figure 7 Single dose of Apa-MS but not Apa-Sol inhibited the in vivo growth of HCC cells and enhanced the effect of RFA on intrahepatic HCC model. Single cells from tumors of solvent control group, RFA group, Apa-Sol group, Apa-MS group, RFA + Apa-Sol group and RFA + Apa-MS group were harvested and injected into liver via hepatic portal vein. After 3–4 weeks, animals were examined using a PET/CT scanner. Quantitative results are shown as (A) PET images of whole animals, (B) photographs of liver with multifocal nodules of HCC, (C) radioactivity ratio of liver to blood and (D) relative nodule number. *P<0.05.
Discussion
Currently, RFA is the prime treatment option for late-stage HCC, particularly for patients with cirrhosis or for those whose liver functional reserve precludes radiotherapy.Citation32 However, HCC can aggressively and rapidly recur after RFA.Citation32 To protect nontumor liver tissue, we cannot indefi-nitely increase the ablation temperature or power during RFA treatment. On the other hand, RFA may induce cellular stress and pathological changes in HCC cells.Citation33,Citation34 Several studies suggested that the elevated EMT may account for post-RFA recurrence.Citation33–Citation35 Therefore, inhibiting the EMT process during RFA treatment may be a useful strategy to overcome the recurrence of HCC after RFA treatment. Apatinib is a novel and effective molecular targeted agent for advanced HCC treatment; it may enhance the effect of RFA treatment and suppress EMT process.Citation36 However, apatinib is insoluble in water, making treatment combination difficult to be applied. To overcome this obstacle, this work aimed to prepare a novel formulation of apatinib that is easily administrated and has elongated effect. This work, for the first time, established a slow releasing system for apatinib based on its insoluble features. The average size of the apatinib microcrystals in the formulation was 30–50 μm. Apatinib microcrystals could not beidentical; however, in our work, the diameter of the particles was relatively uniform. Due to restrictions in the current pharmaceutical techniques, there were many smaller particles. The size of the particle would affect its release, and hence, it is necessary to prepare apatinib microcrystals with different diameters to reveal the relationship between diameter and release behavior. Moreover, our results showed that Apa-MS extended the releasing duration of apatinib as compared with the control formulation. Single-dose administration of Apa-MS enhanced the effect of RFA and had a long-acting effect of inhibiting the EMT process of HCC cells induced by RFA. Apa-MS had several advantages compared with other formulations: 1) Apa-Sol was cleared from tumor tissues within 48 hours, but Apa-MS remained in the tissues for a longer period; 2) only drugs that are soluble could be loaded into microspheres, and Apa-MS exhibited excellent solubility; 3) microcrystals were more tolerant to high temperatures during RFA compared to gelatin sponge particles or liposomes; and 4) Apa-MS released a very high concentration (30–50 mg/mL) of apatinib and permitted injecting a large amount of drug within a small volume. Thus, this work extended our knowledge on apatinib, and injection of Apa-MS into HCC tissues of patients guided by CT or digital subtraction angiography would be a promising combination strategy to enhance the effect of RFA.Citation37
Conclusion
We conclude that the new apatinib formulation Apa-MS allows single-dose apatinib to be slowly released in tumor tissues for up to 9 days and enhances the antitumor effect of RFA on HCC.
Author contributions
All authors made substantial contributions to the design and conception of the study, and acquisition, analysis and interpretation of data, and took part in either drafting or revising the manuscript. All authors gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Abbreviations
| 18F-FDG | = | 18F-radiolabeled fluorodeoxyglucose |
| Apa-MS | = | apa-tinib microcrystal formulation |
| Apa-Sol | = | apatinib solution |
| CT | = | computed tomography |
| EMT | = | epithelial–mesenchymal transition |
| HCC | = | hepatocellular carcinoma |
| PET | = | positron emission tomography |
| qRT-PCR | = | quantitive real-time polymerase chain reaction |
| RFA | = | radiofrequency ablation |
| SCID | = | severe combined immune deficiency |
Acknowledgments
This study was supported in part by the National Natural Science Foundation of China (No 81471761 and No 81501568) and Tianjin Program to Support Science and Technology Research (No 15ZCZDSY00890).
Disclosure
The authors report no conflicts of interest in this work.
References
- WangFSFanJGZhangZGaoBWangHYThe global burden of liver disease: the major impact of ChinaHepatology20146062099210825164003
- BruixJQinSMerlePRESORCE InvestigatorsRegorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trialLancet201738910064566627932229
- LiuZCNingFWangHFEpidermal growth factor and tumor necrosis factor α cooperatively promote the motility of hepatocellular carcinoma cell lines via synergistic induction of fibronectin by NF-κB/p65Biochim Biophys Acta2017186111 Pt A2568258228844984
- JiaHYangQWangTRhamnetin induces sensitization of hepatocellular carcinoma cells to a small molecular kinase inhibitor or chemotherapeutic agentsBiochim Biophys Acta2016186071417143027091611
- LeeMWRamanSSAsvadiNHRadiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a 10-year intention-to-treat analysisHepatology20176561979199028170115
- WangCWangHYangWMulticenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinomaHepatology20156151579159025284802
- XieHYuHTianSWhat is the best combination treatment with transarterial chemoembolization of unresectable hepatocellular carcinoma? A systematic review and network meta-analysisOncotarget201785910050810052329245997
- IkemotoTShimadaMYamadaSPathophysiology of recurrent hepatocellular carcinoma after radiofrequency ablationHepatol Res2017471233026990590
- IwahashiSShimadaMUtsunomiyaTEpithelial-mesenchymal transition-related genes are linked to aggressive local recurrence of hepatocellular carcinoma after radiofrequency ablationCancer Lett20163751475026940140
- TangZKangMZhangBAdvantage of sorafenib combined with radiofrequency ablation for treatment of hepatocellular carcinomaTumori2017103328629128058713
- DongSKongJKongFSorafenib suppresses the epithelial-mesenchymal transition of hepatocellular carcinoma cells after insufficient radiofrequency ablationBMC Cancer20151593926620566
- RoskoskiRJrThe role of small molecule platelet-derived growth factor receptor (PDGFR) inhibitors in the treatment of neoplastic disordersPharmacol Res2018129658329408302
- FornerAReigMBruixJHepatocellular carcinomaLancet2018391101271301131429307467
- KimHAKimKAChoiJIComparison of biannual ultrasonography and annual non-contrast liver magnetic resonance imaging as surveillance tools for hepatocellular carcinoma in patients with liver cirrhosis (MAGNUS-HCC): a study protocolBMC Cancer201717187729268722
- RimolaJDíaz-GonzálezÁDarnellAComplete response under sorafenib in patients with hepatocellular carcinoma: relationship with dermatologic adverse eventsHepatology Epub2017912
- KongYSunLHouZApatinib is effective for treatment of advanced hepatocellular carcinomaOncotarget201786210559610560529285275
- KouPZhangYShaoWSignificant efficacy and well safety of apatinib in an advanced liver cancer patient: a case report and literature reviewOncotarget2017812205102051528103584
- LeeJEKimKLKimDApatinib-loaded nanoparticles suppress vascular endothelial growth factor-induced angiogenesis and experimental corneal neovascularizationInt J Nanomedicine2017124813482228740387
- JeongJHNguyenHKLeeJESuhWTherapeutic effect of apatinib-loaded nanoparticles on diabetes-induced retinal vascular leakageInt J Nanomedicine2016113101310927462154
- BarnesCOKovalevaEGFuXAssessment of microcrystal quality by transmission electron microscopy for efficient serial femto-second crystallographyArch Biochem Biophys2016602616826944553
- ChenYFengFGaoXMiRNA153 reduces effects of chemotherapeutic agents or small molecular kinase inhibitor in HCC cellsCurr Cancer Drug Targets201515317618725714700
- HouJHongZFengFA novel chemotherapeutic sensitivity-testing system based on collagen gel droplet embedded 3D-culture methods for hepatocellular carcinomaBMC Cancer201717172929117859
- HuLDKongDQHuQFPreparation, characterization and in vivo evaluation of injectable long-acting curcumin microcrystalParticul Sci Technol Epub2017310
- LiLKangLZhaoWmiR-30a-5p suppresses breast tumor growth and metastasis through inhibition of LDHA-mediated Warburg effectCancer Lett2017400899828461244
- AllardMKhoudourNRousseauBSimultaneous analysis of regorafenib and sorafenib and three of their metabolites in human plasma using LC-MS/MSJ Pharm Biomed Anal2017142424828494338
- HeYZhouLGaoSDevelopment and validation of a sensitive LC-MS/MS method for simultaneous determination of eight tyrosine kinase inhibitors and its application in mice pharmacokinetic studiesJ Pharm Biomed Anal2017148657228965046
- ZhaoJBaiZFengFCross-talk between EPAS-1/HIF-2α and PXR signaling pathway regulates multi-drug resistance of stomach cancer cellInt J Biochem Cell Biol201672738826783937
- ChaoTITaiWTHungMHA combination of sorafenib and SC-43 is a synergistic SHP-1 agonist duo to advance hepatocellular carcinoma therapyCancer Lett2016371220521326679051
- XuXFanZLiangCA signature motif in LIM proteins mediates binding to checkpoint proteins and increases tumour radiosensitivityNat Commun201781405928094252
- FengFJiangQCaoSPregnane X receptor mediates Sorafenib resistance in advanced hepatocellular carcinomaBiochim Biophys Acta2018186241017103029369785
- FeiYXiongYShenXCathepsin L promotes ionizing radiation-induced U251 glioma cell migration and invasion through regulating the GSK-3β/CUX1 pathwayCell Signal201844627129331585
- LeeHWParkWJHeoYRParkTIParkSYLeeJHTERT-CLPTM1 locus polymorphism (rs401681) is associated with the prognosis of hepatocellular carcinomaOnco Targets Ther2017104853485829042796
- MuaddiHAl-AdraDPBeecroftRLiver transplantation is equally effective as a salvage therapy for patients with hepatocellular carcinoma recurrence following radiofrequency ablation or liver resection with curative intentAnn Surg Oncol201825499199929327179
- TongYYangHXuXEffect of a hypoxic microenvironment after radiofrequency ablation on residual hepatocellular cell migration and invasionCancer Sci2017108475376228182306
- JiangzhengZCaiXHaoXLncRNA FUNDC2P4 down-regulation promotes epithelial-mesenchymal transition by reducing E-cadherin expression in residual hepatocellular carcinoma after insufficient radiofrequency ablationInt J Hyperthermia2018110
- ValleseFMishraNMPagliariMHelicobacter pylori antigenic Lpp20 is a structural homologue of Tipα and promotes epithelial-mesenchymal transitionBiochim Biophys Acta20171861123263327128947343
- AgibaAMNasrMAbdel-HamidSEldinABGeneidiASEnhancing the intestinal permeation of the chondroprotective nutraceuticals glucosamine sulphate and chondroitin sulphate using conventional and modified liposomesCurr Drug Deliv Epub2018122
