Abstract
Background
We report the safety and efficacy of eribulin as a late treatment line in Thai metastatic breast cancer (MBC) patients.
Patients and methods
A total of 30 MBC patients treated with eribulin between January 2014 and January 2017 were retrospectively analyzed. The patients were scheduled to receive 1.4 mg/m2 of eribulin on day 1, day 8 and subsequently every 21 days. All patients had previously received at least three chemotherapy regimens including anthracycline and taxane. Response rate and progression-free survival (PFS) were analyzed.
Results
The median age was 56 years (range, 40–74 years), with a median follow-up time of 5.7 months (range, 0.2–25 months). The overall response rate was 30% (nine patients): four patients had triple-negative breast cancer, three patients had luminal B breast cancer and two patients had luminal A breast cancer. The median PFS was 2.9 months (range, 0.2–14 months). The median number of previous chemotherapy regimens was 4 (range, 3–9). Univariate analysis showed that the number of regimens (four or fewer) prior to eribulin was statistically associated with superior PFS (P = 0.009). Multivariate analysis also showed similar statistical association between number of prior regimens (four or fewer) and better PFS adjusted by age group (≥50 years; hazard ratio = 1.29; 95% CI: 1.0–1.65; P = 0.046). There were no toxic deaths or grade 4 toxicities. Nine (30%) patients had grade 3 anemia toxicities, and the other common toxicities were leukopenia and neutropenia. Four (13%) patients required dose reduction and 16 (53%) patients required dose delay because of toxicities.
Conclusion
Eribulin is an effective drug for heavily pretreated MBC patients with tolerable toxicities. The benefit was superior in patients who received fewer than four previous chemotherapy regimens.
Introduction
Metastatic breast cancer (MBC) is an incurable disease with a limited survival. Only 20%–30% of patients had long term 5-year survival.Citation1 Treatment goals of this disease are to prolong survival and improve quality of life.Citation2 Previous studies of capecitabine, gemcitabine and paclitaxel have demonstrated single-agent chemotherapy response rates.Citation3–Citation5 Eribulin, one of the modern chemotherapeutic agents, is a nontaxane synthetic microtubule dynamic inhibitor and an analog of the halichondrin B derived from marine sponge. Microtubules have an important role in the cell division process, the disturbance of which result in nonreversible mitotic blockage, which causes cellular death. In vitro and in vivo studies involving eribulin have shown a change in transitional morphology between mesenchymal and epithelial phenotypes, epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition of breast cancer cells.Citation25–Citation27 The effect of these changes has resulted in a decrease in migration and invasiveness. Furthermore, eribulin results in a change in abnormal vascularization of the tumor to functional vessels, leading to an improvement in tumor perfusion; therefore, perfusion of the drug into the tumor cells is greater, decreasing the aggressiveness of the cells and reducing metastasis of breast cancer cells.Citation25–Citation27 Eribulin showed an improvement in overall survival of pretreated MBC patients in the Phase III eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE) trial,Citation6,Citation7 and it was approved for use as monotherapy in MBC patients previously given at least two prior chemotherapeutic regimens including anthracycline and taxane.Citation8 To preserve the quality of life and tolerability of patients without compromising efficacy of the treatments, sequential monotherapies are preferable to combination regimens in most clinical MBC scenarios.Citation8 In Thailand, eribulin has been approved for use in MBC patients since 2014. We conducted this retrospective study in pretreated MBC patients to evaluate the clinical benefit and tolerance of the drug in the real-world practice in Thai patients.
Patients and methods
This study is a retrospective review of MBC patients treated with eribulin between January 2014 and January 2017. This study was approved by the ethics committee of Faculty of Medicine, Chiang Mai University, and the committee waived the requirement for consent from patients, as it is a retrospective study. Only de-identified patient data were used in our study. The patients were scheduled to receive 1.4 mg/m2 of eribulin intravenously for 2–5 minutes as a monotherapy on day 1, day 8 and every 21 days with schedule adjustment or dose modification due to intolerable toxicity of the treatment as necessary. All patients had previously received at least three chemotherapy regimens for the advanced disease including at least one of anthracycline or taxane. Patients who received eribulin for at least one cycle were included for analysis. Tumor response rates were measured using response evaluation criteria in solid tumors (RECIST) criteriaCitation9 or by clinical examination. Toxicity was recorded as per National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.0.Citation10 Patients who received at least one dose of eribulin were included for toxicity analysis. Statistical analysis was done using commercial statistical software Stata 11.0 (StataCorp LP, College Station, TX, USA). The results of this study are reported in percentages for descriptive categorical data and as medians with corresponding interquartile ranges for continuous variables. P-values <0.05 were considered as statistically significant. Progression-free survival (PFS) was calculated using the Kaplan–Meier method, and the log-rank test was performed for significance test of the predicted factors that affect the prognosis.
Results
Demographics and baseline clinical characteristics
As shown in , 30 MBC patients having received eribulin were included in the study. Their median age was 56 years (range, 40–74 years). All patients were divided into age range (years) as 36–40 = 1, 41–45 = 0, 46–50 = 5, 51–55 = 8, 56–60 = 10, 61–65 = 3, 66–70 = 2 and 71–75 = 1. Among them, five (17%) were histological type of luminal A, eight (27%) were of luminal B, six (20%) were of HER2 positive and 11 (36%) were of triple negative (TN). Median follow-up time was 5.7 months (range, 0.2–25 months). The median number of previous chemotherapy regimens was 4 (range, 3–9).
Table 1 Patients and cancer characteristics
Efficacy
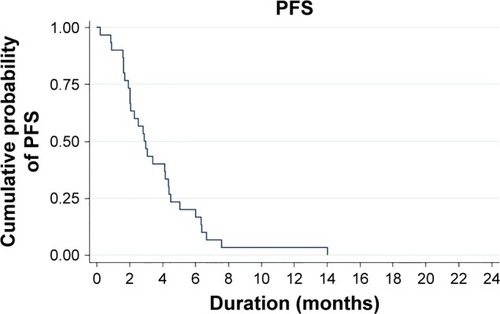
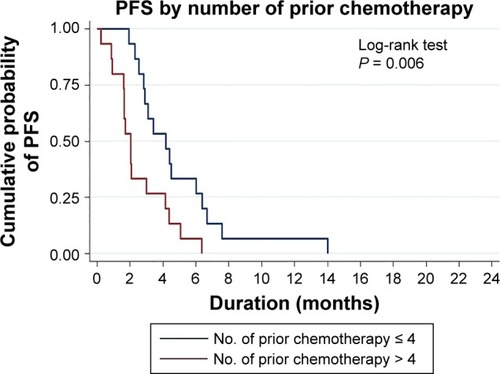
Overall clinical efficacy outcomes following eribulin treatment are shown in . The eribulin efficacy by age range (years) was 36–40 = 1 dead of disease (DD); 41–45 = 0; 46–50 = 4 living with disease (LD), 1 DD; 51–55 = 4 LD, 4 DD; 56–60 = 6 LD, 4 DD; 61–65 =2 LD, 1 DD; 66–70 = 1 LD, 1 DD and 71–75 = 1 DD. The clinical benefit rate (response and stable disease) was 53% (16/30) with partial response seen in nine (30%) patients and stable disease seen in seven (23%) patients. Progressive disease was reported in 14 (47%) patients. Median PFS was 2.9 months (95% CI: 2.0–4.4 months; ). Both our univariate and multivariate analyses showed a statistical association between the number of prior regimens and the PFS. shows that patients with four or fewer previous regimens to have a longer PFS, ie, 4.2 months (median PFS, 95% CI: 2.5–6.0, P-value = 0.009) vs 2.0 months (median PFS, 95% CI: 0.9–3.0). Adjusted by age group (>50 years), the multivariate analysis also showed patients with four or fewer regimens prior to eribulin to have better PFS (hazard ratio = 1.29; 95% CI: 1.0–1.65, P = 0.046). There was no statistical difference of eribulin efficacy between TN and non-TN subtypes.
Table 2 Univariable and multivariable Cox proportional hazard regression analyses
Adverse events (AEs)
We used NCI CTCAE, version 4.0 for grading of toxicities.Citation10 As shown in , most of the patients (20/30) had experienced grade 1 leukopenia (white blood cell [WBC] <3,000/mm3). In all, nine (30%) and three (10%) patients had grade 3 anemia and leukopenia, respectively (grade 3 anemia: hemoglobin 6.5–8.0 g/dL and grade 3 leukopenia: 1,000–2,000/mm3). None of the patients had grade 4 toxicity or fatal AE (grade 4 anemia: hemoglobin <6.5 g/dL, grade 3 leukopenia: hemoglobin <1,000/mm3). There was no statistical association between hematologic toxicities and the numbers of chemotherapies prior to eribulin. Four (13%) patients required dose-reduction from 1.4 to 1.1 mg/m2 and 16 (53%) patients required dose delay because of bone marrow toxicities (). Nonhematologic toxicities were less common in this cohort of patients, and only grade 1 nausea, vomiting and malaise were reported in five (16%) patients. All patients had alopecia from previous chemotherapy when they started eribulin. Patients who experienced myelosuppression, length of this event was between 1 and 21 days (median 14 days).
Table 3 Hematologic AEs
Discussion
Eribulin is approved for use in the treatment of MBC patients who have experienced at least two chemotherapeutic regimens previously, and they should have been pretreated with anthracycline or taxane because of the improvement on overall survival from EMBRACE study.Citation6 Standard dosage of eribulin mesylate is 1.4 mg/m2 for day 1, day 8 and every 21 days in each cycle, but for patient experiencing toxicities, the dose may be delayed or modified to 1.1 or 0.7 mg/m2 depending on severity.Citation6 The most common AEs in our study were hematologic toxicities, which included leukopenia, neutropenia and anemia, which were similar to those of EMBRACE and other studies.Citation6,Citation11–Citation13 However, there was no any grade 4 hematologic toxicities reported in this study. The dose modification was found in 13% of patients and delayed drug administration in 53% of patients due to hematologic toxicities, which were similar to those treated with eribulin in Asian patients as reported in Japan and India.Citation11,Citation14,Citation15 Having shown an improvement in the overall survival in the EMBRACE study,Citation6 eribulin is approved for use in the treatment of MBC patients previously treated with two or more chemotherapeutic regimens. These prior regimens should include anthracycline or taxane. Statistically, we did not find any difference in the response to eribulin between TN subtype breast cancer patients and non-TN breast cancer patients. A median PFS at 2.90 months (95% CI: 2.0–4.4 months) in our study was comparable to that reported in several other studies that used monotherapy in the late line regimens, eg, 4.9 months for capecitabine,Citation16 3.7 months for vinorelbineCitation17 and 3.1 months for ixabepilone.Citation18
When analyzing the impact of number of prior regimens before eribulin, we found that the patients who had received four or fewer regimens had better PFS than those with a higher number of prior regimens, ie, 4 vs 2 months. This finding was not inferior to other regimens.Citation19–Citation22 The EMBRACE study reported a median PFS of 3.7 months for a median number of prior regimens of 4, including neoadjuvant and adjuvant regimens. However, if we focused on the group of patients who received no more than four regimens as in the EMBRACE study, the corresponding median PFS was 4 months. Our study included patients who received at least three prior regimens that might attribute to the poor overall median PFS, but nonetheless in line with 2.5–4 months in other previous reports for heavily pretreated patients (four or more prior lines of treatment).Citation8,Citation11,Citation13,Citation23 The better PFS found in this study associated with fewer prior chemotherapy regimens in this study, which was similar to that reported in previous studies.Citation12,Citation24 Interestingly, four patients of the overall response rate (nine patients) were TN breast cancer, but more patients are needed to evaluate the efficacy between non-TN and TN patients. The reasons behind the more effective outcome of the use of eribulin as an earlier than late treatment line of treatment may be attributed to the nonmitotic effect on tumor biology by reversal of EMT affecting the tumor vasculature remodeling, which will increase tumor oxygenation, leading to a decreased capacity for metastasis and invasion.Citation25–Citation27
Conclusion
Eribulin is an effective drug in heavily pretreated Thai MBC with tolerable toxicities. The benefit was better in patients who received eribulin as an earlier treatment.
Disclosure
The authors report no conflicts of interest in this work.
References
- KubistaEBreast cancer: figures and factsWien Med Wochenschr200115121–23548551 German11762252
- El SaghirNSTfayliAHatoumHANachefZDinhPAwadaATreatment of metastatic breast cancer: state-of-the-art, subtypes and perspectivesCrit Rev Oncol Hematol201180343344921330148
- KinoshitaJHagaSShimizuTMonotherapy with paclitaxel as third-line chemotherapy against anthracycline-pretreated and docetaxel-refractory metastatic breast cancerBreast Cancer20029216616912016397
- RhaSYMoonYHJeungHCGemcitabine monotherapy as salvage chemotherapy in heavily pretreated metastatic breast cancerBreast Cancer Res Treat200590321522115830134
- BursteinHJStornioloAMFrancoSA phase II study of lapatinib monotherapy in chemotherapy-refractory HER2-positive and HER2-negative advanced or metastatic breast cancerAnn Oncol20081961068107418283035
- CortesJO’ShaughnessyJLoeschDEribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised studyLancet2011377976991492321376385
- TwelvesCAwadaACortesJSubgroup analyses from a phase 3, open-label, randomized study of eribulin mesylate versus capecitabine in pretreated patients with advanced or metastatic breast cancerBreast Cancer (Auckl)201610778427398025
- DigkliaAVoutsadakisIAEribulin for heavily pre-treated metastatic breast cancer patientsWorld J Exp Med20155319419926309821
- EisenhauerEATherassePBogaertsJNew response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1)Eur J Cancer200945222824719097774
- ChenAPSetserAAnadkatMJGrading dermatologic adverse events of cancer treatments: the Common Terminology Criteria for Adverse Events Version 4.0J Am Acad Dermatol20126751025103922502948
- BajpaiJRamaswamyAGuptaSGhoshJGuliaSEribulin in heavily pretreated metastatic breast cancer: a tertiary care center experience from IndiaIndian J Cancer201653346046328244486
- FabiAMoscettiLCiccareseMEribulin in heavily pretreated metastatic breast cancer patients and clinical/biological feature correlations: impact on the practiceFuture Oncol201511343143825675124
- PolettiPGhilardiVLivraghiLMilesiLRota CaremoliETondiniCEribulin mesylate in heavily pretreated metastatic breast cancer patients: current practice in an Italian community hospitalFuture Oncol201410223323924490609
- AtesOBabacanTKertmenNEfficacy and safety of eribulin monotherapy in patients with heavily pretreated metastatic breast cancerJ BUON201621237538127273947
- AogiKIwataHMasudaNA phase II study of eribulin in Japanese patients with heavily pretreated metastatic breast cancerAnn Oncol20122361441144821989327
- OsakoTItoYTakahashiSTokudomeNIwaseTHatakeKIntermittent capecitabine monotherapy with lower dose intensity in heavily pretreated patients with metastatic breast cancerTumori200793212913217557557
- SeoHYLeeHJWooOHPhase II study of vinorelbine mono-therapy in anthracycline and taxane pre-treated metastatic breast cancerInvest New Drugs201129236036519943080
- PerezEALerzoGPivotXEfficacy and safety of ixabepilone (BMS-247550) in a phase II study of patients with advanced breast cancer resistant to an anthracycline, a taxane, and capecitabineJ Clin Oncol200725233407341417606974
- YamamuraJMasudaNYamamotoDGemcitabine and vinorelbine combination chemotherapy in taxane-pretreated patients with metastatic breast cancer: a phase II study of the Kinki Multidisciplinary Breast Oncology Group (KMBOG) 1015Chemotherapy201762530731328605730
- BajpaiJRamaswamyAGuptaSGhoshJGuliaSEverolimus in heavily pretreated metastatic breast cancer: is real world experience different?Indian J Cancer201653346446728244487
- UncuDBayogluIVArslanUYTrastuzumab-based retreatment after lapatinib in heavily pretreated HER2 positive metastatic breast cancer: an anatolian society of medical oncology studyAsian Pac J Cancer Prev20151694127413125987098
- HongJYParkYHChoiMKCharacterization of durable responder for capecitabine monotherapy in patients with anthracycline-and taxane-pretreated metastatic breast cancerClin Breast Cancer2015155e287e29225997855
- RamaswamiRO’CathailSMBrindleyJHSilcocksPMahmoudSPalmieriCActivity of eribulin mesylate in heavily pretreated breast cancer granted access via the Cancer Drugs FundFuture Oncol201410336337624367990
- LeeDWTeohDCChongFLTreatment of heavily pre-treated metastatic breast cancer with eribulin: first local experience in SabahMed J Malaysia201671634835028087961
- YoshidaTOzawaYKimuraTEribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) statesBr J Cancer201411061497150524569463
- FunahashiYOkamotoKAdachiYEribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer modelsCancer Sci2014105101334134225060424
- UedaSSaekiTTakeuchiHIn vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumabBr J Cancer2016114111212121827140309