Abstract
Background
Cutaneous squamous cell carcinoma (CSCC) is the second most common carcinoma worldwide. Clinical treatment for patients with CSCC remains non-ideal. Insulin-like growth factor binding protein 3 (IGFBP3), a member of the insulin-like growth (IGF) system, participates in several biological processes, including cellular proliferation and apoptosis. Here, we explored the functional role of IGFBP3 in apoptosis and proliferation of A431 cells, a human CSCC cell line.
Materials and methods
Differential expression analysis, immunohistochemistry, immunoblotting, TUNEL assay, and CCK8 assay techniques were used to investigate the IGFBP3 expression levels in both A431 cells and CSCC tissue surgically obtained from humans as well as to explore the functional role of IGFBP3 in the apoptosis and proliferation of A431 cells.
Results
By using normal epidermal keratinocytes for comparison, we identified the top 10 ranked differentially upregulated genes expressed in human cutaneous squamous cell carcinoma cell lines. Among these 10 genes, IGFBP3 was ranked number 1. By using immunohistochemistry, we found that the expression level of IGFBP3 was significantly elevated in CSCC tissue compared with that in normal human skin tissue. Knockdown of IGFBP3 in A431 cells by transfection with IGFBP3-specific siRNA markedly altered the expression of proteins that contribute to apoptosis via mitochondrial pathways, significantly suppressing the expression of Bax and active caspase-3, while significantly increasing B-cell lymphoma-2 expression. TUNEL assay confirmed the effect of knockdown of IGFBP3 on the apoptosis as well. In addition, knockdown of IGFBP3 inhibited the proliferation of A431 cells.
Conclusion
IGFBP3 is overexpressed in both CSCC cell lines and tissue. Knockdown of IGFBP3 enhanced the apoptosis via a mitochondrial pathway and inhibited the proliferation of A431 cells. These findings indicate that IGFBP3 may be a biomarker and a potential therapeutic target for CSCC.
Keywords:
Introduction
Cutaneous squamous cell carcinoma (CSCC) is a malignant tumor originating from the keratinocytes of the epidermis or an appendage.Citation1 It is the second most common carcinoma globally and is currently thought to be caused by excessive exposure to solar ultraviolet radiation, human papillomavirus, and immunosuppression.Citation2–Citation4 The incidence of CSCC shows a rising trend, especially among elderly white individuals, and CSCC is seriously harmful to health.Citation5,Citation6 Treatment of CSCC includes surgical resection, cryotherapy, photodynamic therapy, and radiotherapy.Citation7 However, there remains a potential for CSCC to recur and metastasize, leading to high morbidity and mortality rates in these patients.Citation8 Therefore, it is important to gain further insight into the pathogenesis of CSCC to develop better treatment methods.Citation9
Increasing evidence indicates that the insulin-like growth factor (IGF) system is critical in the processes of tumor occurrence, development, and transformation. The IGF system comprises two peptides (IGF1 and IGF2), their receptors, and six IGF binding proteins (IGFBPs), of which IGFBP3 is the most abundant in the circulation and plays an essential role in regulating the IGF signaling pathways.Citation10,Citation11 Recent studies have shown that IGFBP3 sequesters IGF1 from its receptor to inhibit its action and to promote cell apoptosis of non-small cell lung cancer, colon cancer, breast cancer, esophageal cancer, and prostate cancer cells.Citation12–Citation16 Although IGFBPs have been shown to have IGF-independent actions, these activities are not well understood.Citation11,Citation17 Methylation of IGFBP3 inhibits its expression in colorectal, gastric, and breast carcinomas.Citation18 Furthermore, there is evidence that a link between IGFBP3 and p53 increases apoptosis of cells.Citation19 Conversely, it has been suggested that IGFBP3 induces growth factor-β1 and epidermal growth factor receptor and leads to the proliferation of tumor cells.Citation20,Citation21 Studies have also demonstrated that glycosylation of IGFBP3 is closely correlated with the occurrence and development of breast cancer and is associated with a worse prognosis for patients with this disease.Citation17 Furthermore, several studies have reported that the upregulation of IGFBP3 expression may be linked to lymph node metastasis of tumors.Citation22,Citation23 However, other studies have shown that IGFBP3 has dual functional effects on apoptosis and proliferation in malignant melanoma and astrocytoma.Citation24,Citation25 Consequently, the role of IGFBP3 in tumor development is complex and controversial and has thus become the focus of recent research.Citation24 At present, little is known regarding its role in CSCC.
In this study, we first used the Gene Expression Omnibus (GEO) database developed by the National Center for Biotechnology Information (NCBI) to investigate differentially expressed genes (DEGs) in human CSCC cell lines. On the basis of our findings, we next explored the expression profile of IGFBP3 in CSCC tissue surgically derived from patients. Finally, we determined whether IGFBP3 played a functional role in apoptosis and proliferation in a human CSCC cell line (A431), which would suggest its potential use as a biomarker in the early diagnosis or treatment of CSCC.
Materials and methods
Materials
Specific siRNAs for human IGFBP3 (5′-GCACAGAUACCCAGAACUUUU-3′) and scrambled siRNA (5′-UAACGACGCGACGACGUAA-3′) were designed and obtained from Biomics Biotechnologies Co., Ltd. (Nantong Shi, Jiangsu Sheng, China). Lipofectamine 3000 was purchased from Thermo Fisher Scientific (Waltham, MA, USA) for cell transfection. Anti-β-tubulin, anti-Bax, anti-caspase 3, anti-B-cell lymphoma-2 (Bcl-2), and anti-IGFBP3 primary antibodies were purchased from Bioss Biotechnology (Beijing, China).
Microarray data
The transcription profile of GSE66359 was downloaded from the NCBI GEO database (http://www.ncbi.nlm.nih.gov/geo/). The data included 13 samples from normal human epidermal keratinocytes (n = 5, GSM1620805 to GSM1620809) and CSCC cell lines (n = 8, GSM1620810 to GSM1620817).
Differential gene expression analysis
The Limma package in R software (http://www.r-project.org/) was used to conduct all statistical analyses. Upregulated and downregulated DEGs were defined and acquired as a fold-change threshold greater than 1.5. The R software was also used to perform a clustering analysis of the obtained DEGs and to generate a heat map.
Cell culture and transfection
The human CSCC cell line A431 (American Type Culture Collection [ATCC], Manassas, VA, USA) was cultured in Dulbecco’s Modified Eagle’s Medium (4.5 g/L glucose) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 mg/L streptomycin in an incubator under conditions of saturated humidity, 37°C, and 5% carbon dioxide. Cells in the logarithmic growth phase were used for experiments. The A431 cells in 12-well plates were transfected with IGFBP3 or with scrambled siRNA using Lipofectamine 3000 in medium without fetal bovine serum. Functional studies were conducted 48 hours after transfection.
Western blotting
Immunoblotting was performed as previously described.Citation26 In brief, A431 cells were lysed using a detergent extraction buffer that contained 150 mmol/L NaCl, 20 mmol/L Tris-HCl (pH 7.5), 1 mmol/L EDTA, 1 mmol/L EGTA, 1% Nonidet P-40, 2.5 mmol/L sodium pyrophosphate, and 1% sodium deoxycholate, as well as protease inhibitor cocktail tablets. Protein concentrations were determined using the Bradford assay. Extracted proteins were loaded onto 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels and separated using a voltage of 100 V for 1 hour. The separated proteins were transferred to polyvinylidene difluoride membranes. Non-specific sites were blocked using PBS with 0.1% Tween 20 and 5% non-fat milk for 1 hour at room temperature on a rocking platform. Primary antibodies (IGFBP3: bs-1434R; Bcl-2: bs-0032R; Bax: bs-0127R; active caspase-3: bs-0081R; β-tubulin: bs-4511R; Bioss Company, 1:200) were incubated overnight at 4°C. After washing with PBS, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies (1:1,000). Antibody binding was detected using an enhanced chemiluminescence system. The intensity of each band was analyzed and is represented as the relative intensity of the anti-β-tubulin antibody. The data were expressed in percentage of the intensity between targeted proteins divided by β-tubulin.
Immunohistochemistry
This study was approved by the Clinical Research Ethics Committee at Anhui Medical University. Each specimen was obtained from a patient who provided written informed consent. All procedures conformed to the Declaration of Helsinki and Good Clinical Practice.Citation27,Citation28
The surgically obtained human CSCC tissues were fixed with 4% paraformaldehyde for 24–48 hours, dehydrated, embedded in paraffin, and sliced into 5-μm thick sections. The sections were then deparaffinized and rehydrated using routine methods. Antigen retrieval was accomplished by heating the sections in citrate buffer solution (0.01 M, pH 6.0) with a microwave oven. The sections were rinsed and then treated for 30 minutes with 3% hydrogen peroxide in deionized water to remove endogenous peroxidase activity. Non-specific binding sites were blocked by incubating in 5% normal goat serum for 30 minutes at room temperature. Afterward, the slides were incubated with rabbit anti-IGFBP3 primary antibody at 4°C overnight. After rinsing with PBS, the slides were incubated with biotin-labeled goat anti-rabbit IgG secondary antibody. Streptavidin–horseradish peroxidase was applied at 37°C for 30 minutes. The sections were washed with PBS, incubated with diaminobenzidine for 10 minutes (the degree of staining was controlled by visualization under a light microscope), and then washed with distilled water. Hematoxylin was used to stain cell nuclei. Dehydration and clearing of tissue sections were conducted as needed using standard protocols. Brown particles stained in cells were regarded as positive for the presence of antibody. The primary antibody was replaced by normal rabbit serum to provide a negative control. The experiments were repeated in specimens from six patients, and the data were evaluated by pathologists of Anhui Provincial Hospital.
TUNEL assay
Experiments were performed according to our previous study.Citation29 TUNEL signal was detected via a kit following the manufacturer’s instructions. Images of TUNEL assay were captured using fluorescence microscopy, and the data were analyzed with ImageJ software.
CCK8 assay
According to the previous study, the viability of the A431 cells was identified by the CCK8 assay.Citation30 After the cells were transfected with scrambled or IGFBP3 siRNA for 48 hours, A431 cells were trypsinized and seeded into 96-well plates at an equal density of 6 × 103 cells/well. On the next day, 10 μL of CCK8 was added to each well of the 96-well plate and incubated for 4 hours at 37°C with 5% CO2. Eventually, the absorbance of the solution of each well was determined at a wavelength of 450 nm. The cell viability is expressed as the absorbance value (optical density: OD).
Statistical analysis
We used SigmaPlot software (Systat Software Inc, San Jose, CA, USA) to perform unpaired, two-tailed Student’s t-tests. All data are expressed as mean ± standard error of the mean (SEM). P<0.05 was considered statistically significant.
Results
Identification of DEGs
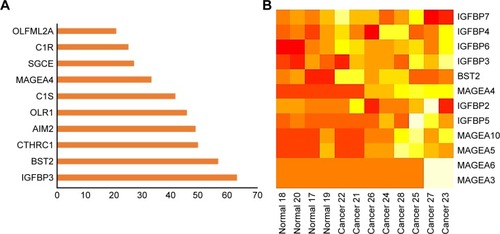
Microarray expression profiling was conducted using the GEO database to identify significant DEGs in CSCC cell lines compared with normal human epidermal keratinocytes. On the basis of a fold change greater than 1.5, we identified the top 10 upregulated DEGs (). Of these, IGFBP3 was ranked number one. shows the heat map generated for the expression profiles of IGFBPs, bone marrow stromal protein 2 (BST2), and members of the melanoma antigen family A across different normal and CSCC cell samples.
Figure 1 Rank order and heat map of genes differentially expressed in cutaneous squamous cell carcinoma cells vs normal human epidermal keratinocytes.
Abbreviations: AIM2, absent in melanoma 2; BST2, bone marrow stromal protein 2; C1R, complement component one subcomponent r; C1S, complement component one subcomponent s; CTHRC1, collagen triple helix repeat containing 1; IGFBP, insulin-like growth factor binding protein; MAGEA, melanoma antigen family A; OLR1, oxidized low-density lipoprotein receptor 1; OLFML2A, olfactomedin-like 2A; SGCE, epsilon-sarcoglycan gene.
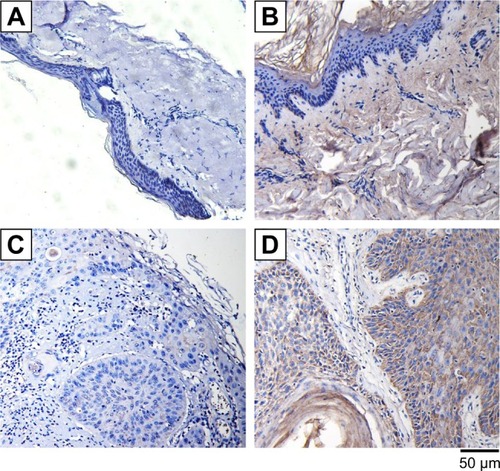
Enhanced expression of IGFBP3 in human CSCC tissue
Because growing evidence has indicated that IGFBP3 is involved in the development of various cancers, including colon cancer, breast cancer, and malignant melanoma,Citation13,Citation14,Citation24 we also investigated IGFBP3 expression in surgically obtained specimens from patients with CSCC. The results of our immunohistochemistry assays indicated that brown signal representing IGFBP3 protein was markedly stronger in the cells of CSCC tissue than that in the cells of normal skin tissue (). But the brown signals were not found if the primary antibody was replaced by normal rabbit serum as the negative control (). On the basis of this finding, we speculated that IGFBP3 may also contribute to the development of CSCC.
Figure 2 Expression levels of IGFBP3 in normal human skin and cutaneous squamous cell carcinoma tissues.
Abbreviation: IGFBP3, insulin-like growth factor binding protein-3.
Functional role of IGFBP3 in the apoptosis and proliferation of CSCC cells
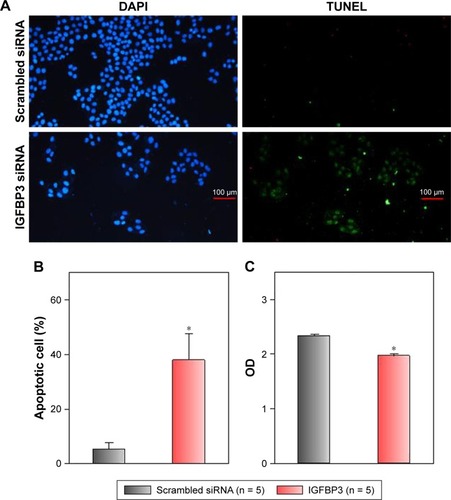
Recent studies have shown that IGFBP3 is a regulator of cancer cell apoptosis.Citation12–Citation16 To explore the functional role of IGFBP3 in the apoptosis of CSCC cells, we used IGFBP3-specific siRNA to inhibit IGFBP3 expression. We first showed that compared with the expression of IGFBP3 following transfection of A431 cells with scrambled control siRNA, the expression of IGFBP3 following transfection with IGFBP3-specific siRNA was substantially reduced (). Thus, we next used western blot assays to examine the change in the expression of apoptosis-related proteins. Our results indicated that IGFBP3 knockdown through transfection with IGFBP3-specific siRNA in A431 cells significantly suppressed the expression of Bax and active caspase-3, suggesting a pro-apoptotic effect; however, the expression of Bcl-2, which is one of the most important anti-apoptotic proteins, was significantly increased (). To confirm our results further, we used TUNEL assay to detect apoptotic cells. Our data showed that IGFBP3 siRNA markedly enhanced the percentage of apoptotic A431 cells (). Moreover, the role of IGFBP3 in the proliferation of A431 cells was also investigated via CCK8 assay. We found that IGFBP3 siRNA significantly reduced A431 cell proliferation compared to scrambled siRNA control (). Together, these data suggested the involvement of IGFBP3 in apoptosis and proliferation of human CSCC A431 cells.
Figure 3 Effects of IGFBP3-specific siRNA on IGFBP3 expression in A431 cells.
Abbreviations: IGFBP3, insulin-like growth factor binding protein-3; SEM, standard error of the mean.
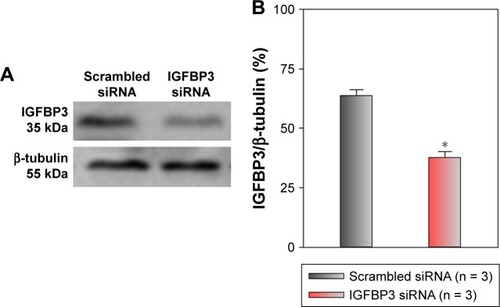
Figure 4 Effects of IGFBP3-specific siRNA on Bcl-2, Bax, and active caspase-3 expression levels in A431 cells.
Abbreviations: Bcl-2, B-cell lymphoma-2; IGFBP3, insulin-like growth factor binding protein-3; SEM, standard error of the mean.
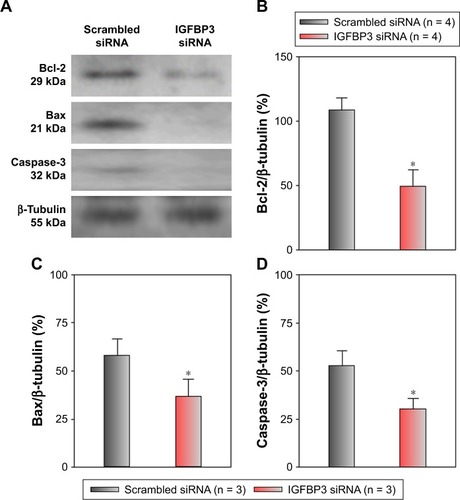
Figure 5 Effects of IGFBP3-specific siRNA on apoptosis and proliferation of A431 cells.
Abbreviations: IGFBP3, insulin-like growth factor binding protein-3; SE, standard error.
Discussion
IGFBP3 plays complex roles in several types of cancers. However, the function of IGFBP3 in the regulation of CSCC remains elusive. In this study, we investigated the expression profile of IGFBP3 in CSCC and the functional role of IGFBP3, a member of the IGF system, in the apoptosis and proliferation of A431 cells. We initially found that IGFBP3 expression was ranked first in the upregulated DEGs of CSCC cell lines compared with normal human epidermal keratinocytes. We next determined that compared with normal skin tissue, the expression level of IGFBP3 was enhanced in CSCC tissue. Finally, our results indicated that knockdown of IGFBP3 in a CSCC cell line significantly enhanced the apoptosis and proliferation.
CSCC is the second most common malignant neoplasm, and evidence indicates that its incidence is increasing.Citation2,Citation5,Citation6 Unfortunately, CSCC has the potential to recur and metasta-size following initial the treatment of the primary malignant neoplasm, leading to substantial morbidity and mortality.Citation7,Citation8 Thus, identification of the pathogenic mechanisms and discovery of novel treatment strategies, such as targeted therapy for CSCC, are critically important.Citation9 IGFBP3 is the most abundant IGFBP in the IGF system, which plays essential roles in regulating cell proliferation and apoptosis, even showing dual functional effects in several types of cancer cells.Citation10,Citation25 Of the top 10 ranked upregulated DEGs in CSCC cell lines (as defined by greater than 1.5-fold changes in expression levels), IGFBP3 was ranked highest. Our identification of the DEGs showed that the level of IGFBP3 expression was significantly increased in CSCC. To explore this finding further, we used surgically derived specimens from patients with CSCC and found that IGFBP3 was also elevated in these tissues, as indicated by immunohistochemical staining. This finding led us to believe that IGFBP3 may be associated with the regulation of CSCC. Apoptosis is a complex process that involves several signaling pathways.Citation31,Citation32 Cysteinyl aspartate-specific proteases (caspase) are key mediators of apoptosis,Citation33 and caspase family members are classified as cytokine processors (caspase 1, 4, 5, 11, 12, 13, and 14), apoptotic initiators (caspase 2, 8, 9, and 10), and apoptotic executioners (caspase 3, 6, and 7). Caspase 3 is significantly associated with cell death via activation by initiators (mainly caspase 8 and 10).Citation34 Members of the Bcl-2 family are also key apoptotic regulators, including anti-apoptotic proteins (mainly Bcl-2) and pro-apoptotic proteins (mainly Bax).Citation35 A variety of studies have demonstrated that IGFBP3 is involved with cell apoptosis and proliferation.Citation12–Citation16 Therefore, we explored the regulation of IGFBP3 in the apoptosis and proliferation of A431 cells. We found that IGFBP3 knockdown not only markedly increased the expression of Bcl-2 but also significantly suppressed the expression levels of Bax and active caspase 3. Moreover, TUNEL assay also confirmed that IGFBP3 siRNA markedly enhanced the apoptosis of A431 cells. On the other hand, we used CCK8 assay to find that IGFBP3 siRNA significantly inhibited the proliferation of A431 cells. These data indicated that IGFBP3 is involved in the apoptosis and proliferation of A431 cells and suggested that IGFBP3 may be a therapeutic target for CSCC.
Regarding the remaining top 10 upregulated DEGs in CSCC cells (shown in ), we identified BST2, which is consistent with reports that it is overexpressed in breast cancer cells.Citation36–Citation38 Similarly, collagen triple helix repeat containing 1 was also identified and has been found to be induced in melanoma, colorectal cancer, and non-small cell lung cancer.Citation39–Citation41 However, the expression of absent in melanoma 2, which was also 1 of the top 10 DEGs in this study, is decreased in breast cancer and prostate cancer cells compared with normal cells.Citation42,Citation43 Oxidized low-density lipoprotein receptor 1 promotes cancer growth and migration and inhibits apoptosis.Citation44,Citation45 Melanoma antigen family A4 has important biological functions in the majority of serious carcinomas.Citation46,Citation47 Epsilon-sarcoglycan gene mutations cause neurological disorders such as myoclonus-dystonia syndrome.Citation48 Olfactomedin-like 2A is a senescence-specific gene and helps during chemotherapy of carcinoma.Citation49 Complement components 1r and 1s are highly specific serine proteases that promote the progression and metastasis of melanoma, breast, lung, colon, and pancreatic cancers.Citation50 All these genes and gene products warrant further investigation in future studies examining CSCC.
Conclusion
We demonstrated that IGFBP3 was upregulated in CSCC tissues and that knockdown of IGFBP3 enhanced the apoptosis via the mitochondrial pathway and inhibited proliferation of A431 cells. Taken together, our results suggest that IGFBP3 may be a biomarker and a potential therapeutic target for CSCC. However, the effect of IGFBP3 on the migration, invasion, and other progress of CSCC remains unknown. Further study will be required to determine the function of IGFBP3 in the regulation of CSCC.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (grant numbers U1732157, 81701879, and 81570403); National Training Program of Innovation and Entrepreneurship for Undergraduates (grant number 201610366003); Anhui Provincial Natural Science Foundation (grant number 1708085MH187); Anhui Medical University for Scientific Research (grant number BSKY XJ201607); and Outstanding Young Investigator of Anhui Medical University.
Disclosure
The authors report no conflicts of interest in this work.
References
- MadanVLearJTSzeimiesRMNon-melanoma skin cancerLancet2010375971567368520171403
- ParekhVSeykoraJTCutaneous Squamous Cell CarcinomaClin Lab Med201737350328802498
- SaladiRNPersaudANThe causes of skin cancer: a comprehensive reviewDrugs Today20054113715753968
- MittalAColegioORSkin Cancers in Organ Transplant RecipientsAm J Transplant201717102509253028556451
- StaplesMMarksRGilesGTrends in the incidence of non-melanocytic skin cancer (NMSC) treated in Australia 1985–1995: Are primary prevention programs starting to have an effect?Int J Cancer19987821441489754642
- GrayDTSumanVJSuWPTrends in the population-based incidence of squamous cell carcinoma of the skin first diagnosed between 1984 and 1992Arch Dermatol199713367357409197827
- AlamMRatnerDAlamMRatnerDCutaneous squamous-cell carcinomaN Engl J Med2001344344975975983983
- Garcia-ZuazagaJOlbrichtSMCutaneous squamous cell carcinomaAdv Dermatol200824335719256304
- KivisaariAKähäriVMSquamous cell carcinoma of the skin: Emerging need for novel biomarkersWorld J Clin Oncol201344859024926428
- RankeMBInsulin-like growth factor binding-protein-3 (IGFBP-3)Best Pract Res Clin Endocrinol Metab201529570171126522455
- ButtAJFirthSMKingMABaxterRCInsulin-like growth factor-binding protein-3 modulates expression of Bax and Bcl-2 and potentiates p53-independent radiation-induced apoptosis in human breast cancer cellsJ Biol Chem2000275503917410998426
- GeorgesRBAdwanHHamdiHThe insulin-like growth factor binding proteins 3 and 7 are associated with colorectal cancer and liver metastasisCancer Biol Ther2011121697921525788
- ShiratsuchiIAkagiYKawaharaAExpression of IGF-1 and IGF-1R and their relation to clinicopathological factors in colorectal cancerAnticancer Res20113172541254521873172
- YerushalmiRGelmonKALeungSInsulin-like growth factor receptor (IGF-1R) in breast cancer subtypesBreast Cancer Res Treat2012132113114221574055
- MccollKESerum IGF-1 linking visceral obesity with esophageal adenocarcinoma: unconvincing evidenceAm J Gastroenterol2012107220520622306944
- DaragóASapotaAMatychJThe correlation between zinc and insulin-like growth factor 1 (IGF-1), its binding protein (IGFBP-3) and prostate-specific antigen (PSA) in prostate cancerClin Chem Lab Med20114910169921671822
- BaricevićIMasnikosaRLagundžinDGolubovićVNedićOAlterations of insulin-like growth factor binding protein 3 (IGFBP-3) glycosylation in patients with breast tumoursClin Biochem201043972573120307522
- TomiiKTsukudaKToyookaSAberrant promoter methylation of insulin-like growth factor binding protein-3 gene in human cancersInt J Cancer2007120356657317096329
- BuckbinderLTalbottRVelasco-MiguelSInduction of the growth inhibitor IGF-binding protein 3 by p53Nature199537765506466497566179
- KansraSEwtonDZWangJFriedmanEIGFBP-3 mediates TGF beta 1 proliferative response in colon cancer cellsInt J Cancer200087337310897042
- MartinJLde SilvaHCLinMZScottCDBaxterRCInhibition of insulin-like growth factor-binding protein-3 signaling through sphingosine kinase-1 sensitizes triple-negative breast cancer cells to EGF receptor blockadeMol Cancer Ther201413231624337110
- ZhongLPYangXZhangLOverexpression of insulin-like growth factor binding protein 3 in oral squamous cell carcinomaOncol Rep2008206144119020726
- HanselDERahmanAHouseMMet proto-oncogene and insulin-like growth factor binding protein 3 overexpression correlates with metastatic ability in well-differentiated pancreatic endocrine neoplasmsClin Cancer Res20041018 Pt 16152615815448002
- OyGFSlipicevicADavidsonBBiological effects induced by insulin-like growth factor binding protein 3 (IGFBP-3) in malignant melanomaInt J Cancer2010126235036119588500
- SantoshVArivazhaganASreekanthreddyPGrade-specific expression of insulin-like growth factor-binding proteins-2, -3, and -5 in astrocytomas: IGFBP-3 emerges as a strong predictor of survival in patients with newly diagnosed glioblastomaCancer Epidemiol Biomarkers Prev2010196139920501753
- MaYZhangPLiJEpoxyeicosatrienoic acids act through TRPV4-TRPC1-KCa1.1 complex to induce smooth muscle membrane hyperpolarization and relaxation in human internal mammary arteriesBiochim Biophys Acta20151852355255925511389
- World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjectsJAMA2013310202191219424141714
- GrimesDAHubacherDNandaKThe Good Clinical Practice guideline: a bronze standard for clinical researchLancet2005366948017216005342
- XueHLuJYuanRKnockdown of CLIC4 enhances ATP-induced HN4 cell apoptosis through mitochondrial and endoplasmic reticulum pathwaysCell Biosci20166526816615
- KaiSLeiHWenxiuHBingSAmanXChloride intracellular channel 4 protein promotes gastric cancer cell proliferation, invasion and migrationInt J Clin Exp Pathol2016917701775
- PerteaMKimDPerteaGMLeekJTSalzbergSLTranscript-level expression analysis of RNA-seq experiments with HISAT, StringTie and BallgownNat Protoc2016119165027560171
- SarvothamanSUndiRBPasupuletiSRGuttiUGuttiRKRole in myeloid cell developmentApoptosis2015507379
- NicholsonDWThornberryNACaspases: killer proteasesTrends Biochem Sci19972282993069270303
- WolfBBGreenDRSuicidal tendencies: apoptotic cell death by caspase family proteinasesJ Biol Chem199927429200492005210400609
- WongWWLPuthalakathHBcl-2 family proteins: The sentinels of the mitochondrial apoptosis pathwayIUBMB Life2013606390397
- CaiDUp-regulation of bone marrow stromal protein 2 (BST2) in breast cancer with bone metastasisBmc Cancer2009911019118499
- YiEHYooHNohKHBST-2 is a potential activator of invasion and migration in tamoxifen-resistant breast cancer cellsBiochem Biophys Res Commun2013435468569023702480
- JonesPHMahauad-FernandezWDMadisonMNOkeomaCMBST-2/tetherin is overexpressed in mammary gland and tumor tissues in MMTV-induced mammary cancerVirology20134441–212423806386
- TangLDaiDLSuMAberrant expression of collagen triple helix repeat containing 1 in human solid cancersClin Cancer Res200612123716372216778098
- KimHCKimYSOhHWCollagen triple helix repeat containing 1 (CTHRC1) acts via ERK-dependent induction of MMP9 to promote invasion of colorectal cancer cellsOncotarget20145251952924504172
- KeZHeWLaiYOverexpression of collagen triple helix repeat containing 1 (CTHRC1) is associated with tumour aggressiveness and poor prognosis in human non-small cell lung cancerOncotarget20145199410942425238260
- ChenIFOu-YangFHungJYAIM2 suppresses human breast cancer cell proliferation in vitro and mammary tumor growth in a mouse modelMol Cancer Ther200651116432157
- PonomarevaLLiuHDuanXAIM2, an IFN-inducible cytosolic DNA sensor, in the development of benign prostate hyperplasia and prostate cancerMol Cancer Res201311101193120223864729
- HirschHAIliopoulosDJoshiAA transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseasesCancer Cell201017434820385360
- KhaidakovMMitraSKangBYOxidized LDL receptor 1 (OLR1) as a possible link between obesity, dyslipidemia and cancerPLoS One201165e2027721637860
- DhodapkarMVOsmanKTeruya-FeldsteinJExpression of cancer/testis (CT) antigens MAGE-A1, MAGE-A3, MAGE-A4, CT-7, and NY-ESO-1 in malignant gammopathies is heterogeneous and correlates with site, stage and risk status of diseaseCancer Immun20033912875607
- YakirevichESaboELavieOExpression of the MAGE-A4 and NY-ESO-1 cancer-testis antigens in serous ovarian neoplasmsClin Cancer Res2003917645314695148
- PeallKJSmithDJKurianMASGCE mutations cause psychiatric disorders: clinical and genetic characterizationBrain2013136Pt 129430323365103
- SchwarzeSRFuVXDesotelleJAKenowskiMLJarrardDFThe identification of senescence-specific genes during the induction of senescence in prostate cancer cellsNeoplasia20057981616229804
- Afshar-KharghanVThe role of the complement system in cancerJ Clin Invest2017127378028248200
