Abstract
Patients with Lynch Syndrome (LS) are at high risk of developing colorectal cancer at an early age. Germline mutations in DNA mismatch repair genes and microsatellite instability are clear signatures of this autosomal dominant disorder. Here, we report the clinical history of a 38-year-old patient with LS-related metastatic colon cancer treated in Chile with immunotherapy (pembrolizumab). The patient exhibited a pathogenic deletion in Epithelial cell Adhesion Molecule (EPCAM) and mutS homolog 2 (MSH2) genes, and after diagnosis received 12 cycles of FOLFOX. The tumor mass, however, continued to grow, and a new metastatic mucinous adenocarcinoma of 13 mm appeared at the level of the 11th right dorsal vertebra. To treat these lesions, the patient received immunotherapy scheme with pembrolizumab (200 mg every 21 days). After only four cycles, the patient’s symptoms improved and the lesions showed less metabolic activity. After 12 cycles with pembrolizumab, the patient started palliative radiation and systemic second-line treatment with FOLFIRI and Avastin. The immunotherapy scheme with pembrolizumab was capable of delaying the second-line treatment for at least 8 months, becoming a useful therapeutic option for this patient. Thus, our study highlights the importance of implementing immunotherapy treatment programs for LS-colorectal cancer patients in South American countries.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Mutations in genes that encode for DNA mismatch repair (MMR) proteins results in a systematic accumulation of DNA replication errors. In time, these mutations may evolve leading to tumor development. Short repetitive nucleotide sequences, known as microsatellite, are susceptible targets for these replication errors, a condition called microsatellite instability (MSI).Citation1,Citation2 Loss of MMR protein functions results in hereditary nonpolyposis colorectal cancer (HNPCC).Citation3 However, when HNPCC develops as a consequence of germline mutations in at least one of the genes that encode proteins implicated in the MMR system, the pathology is classified as Lynch syndrome (LS). Thus, mutations in mutS homolog 2 (MSH2), mutS homolog 3 (MSH3), mutS homolog 6 (MSH6), mutL homolog 1 (MLH1), PMS1 homolog 1, mismatch repair system component (PMS1, also known as MLH2), and PMS1 homolog 2, mismatch repair system component (PMS2, also known as MLH4) are usually involved in LS.Citation3–Citation10 LS is considered the most common condition among the inherited colorectal cancers and is responsible for 2%–4% of all colorectal carcinomas (CRC).Citation3,Citation4,Citation7,Citation11–Citation13 This syndrome corresponds to an autosomal dominant disorder. Therefore, first-degree relatives have up to 50% risk of inheriting the mutation, and depending on the mutation penetrance, several members of their family may also be clinically affected. Thus, patient’s clinical information is complemented with family history according to the Amsterdam criteria and the Bethesda guidelines.Citation14 LS patients are at high risk of developing CRC (up to 75%), endometrial cancer (32%–45%), stomach cancer (13%–19%), and ovarian cancer (9%–14%).Citation3,Citation5,Citation14,Citation15 But, the risk of developing cancer in other tissues such as small intestine, pancreas, biliary and urinary tract, brain (glioblastomas), skin (keratoacanthomas), and sebaceous tissues are also high.Citation3,Citation5,Citation7,Citation9,Citation12,Citation13,Citation16 Furthermore, cancer develops at a relatively young age in LS patients, and in the case of CRC, the neoplasm progression is rather fast.Citation17
In LS deletions, frameshifts, nonsense, missense, and splice mutations are frequent in MSH2 and MLH1 genes, whereas mutations in MSH6 and PMS2 represent <10%.Citation3,Citation7,Citation9 MSH2 loss of function impairs the recognition of base–base mismatch and insertion–deletion loops, which depends on the heterodimer formation between MSH2 and MSH6 or MSH2 and MSH3. Meanwhile, the repair of base–base mismatches and insertion–deletion loops depends mainly on MLH1, PSM1, and PMS2 proteins.Citation18
Another gene implicated in LS is Epithelial cell Adhesion Molecule (EPCAM; OMIM#185535, previously known as TACSTD1), whose deletion produces allele-specific methylation of MSH2 (OMIM#609309) gene promoter, precluding MSH2 expression.Citation3–Citation5,Citation12 Despite the broad spectrum and particular frequency of pathogenic MMR variants in the Latin America population, only a few cases have been described.Citation14
According to FIRE 3 and TRIBE trials, patients with metastatic CRC (mCRC) or nonresectable CRC have an overall survival of 28.7–37.1 months.Citation19,Citation20 Those patients can follow a first-line treatment, which consists of chemotherapy (either infused fluoropyrimidine plus oxaliplatin or irinotecan) in combination with a biological agent. Both chemotherapy regimens have similar efficiency. Therefore, the selection of either of the chemotherapy regimens depends on patient’s differential toxicity profile. Likewise, the choice of the biological agent will depend on patient’s molecular profile and the absence of contraindications.Citation21 The second-line treatment depends on patient’s organ function and cytotoxicity tolerance and the first-line therapy choice. A third-line treatment considers cetuximab or panitumumab for RAS and BRAF wild-type patients and EGFR antibody for patients who have not used that antibody previously. Regorafenib (multikinase inhibitor) or Trifluridine/tipiracil is recommended for RAS wild-type patients treated with EGFR antibodies and in patients pretreated with fluoropyrimidines, oxaliplatin, irinotecan, and bevacizumab.Citation22
Patients with LS show flat tumors located in the proximity of splenic flexure with poor differentiation and lymphocytic infiltration.Citation19 Likewise, these tumors present a microenvironment populated by myeloid cells expressing the programmed death ligand 1 (PD-L1), which impair the function of the infiltrated CD8+ T and T-helper 1 cells. The immunological blockade can be bypassed, however, by using monoclonal antibodies against PD-1. The usage of PD-1 antibodies has shown promising results in other cancers such as melanoma and non-small cell lung cancer.Citation23–Citation27 Surprisingly, in the case of CRC patients, anti-PD-1 immunotherapy shows different outcomes. Thus, patients whose tumors exhibit mismatch repair-deficiencies are more likely to respond than those whose tumors lack mutations in mismatch repair genes.Citation28,Citation29 This finding opened a new therapeutic door to treat LS patients based on the molecular signature rather than the type of tumor.Citation30 In this study, we report a male with LS-related colon cancer, who exhibits pathogenic deletion involving both EPCAM and MSH2. The patient, treated in Chile, received an immunotherapy scheme with pembrolizumab (Keytruda) that delayed the second-line treatment with FOLFIRI and Avastin for at least 8 months. This emphasizes the importance of including LS-colorectal cancer patients in immunotherapy treatment programs in Latin America.
Case report
The patient gave written informed consent to publish this case report and associated images. The clinical history of the following report corresponds to a patient aged 38 years, at the time of diagnosis. He had a car accident in 2004, which resulted in secondary transient erythroblastopenia with recovered hemiparesis. In January 2015, he underwent surgery to remove his appendix due to acute appendicitis. A biopsy was performed detecting an adenocarcinoma including serous tissue. An analysis of the family history revealed that his father had been diagnosed with colon cancer and skin carcinoma. At the end of March, the patient was referred to Instituto Oncologico Fundación Arturo López Pérez in Chile for further analysis. A blood test revealed an elevated level of the carcinoembryonic antigen (CEA of 237), and an exploratory laparotomy found tumors in the ascending colon and retroperitoneal space. An R0 surgery was planned, and to that end, positron emission tomography-computed tomography (PET/CT) scan was used to define the surgery area to the right side of the colon. The R0 surgery removed the parietal peritoneum including tumor masses from the retroperitoneum.
The analysis of the biopsy showed a poorly differentiated mucinous adenocarcinoma of 10 cm in the cecum and a second poorly differentiated tubular adenocarcinoma in the appendix implantation base with an increased lymphatic vessel permeability and infiltration until adventitia. A third 2 cm tumor mass was found in the ileocecal valve corresponding to a poorly differentiated mucinous adenocarcinoma with 21/31 positive lymph nodes. Likewise, the retroperitoneal mass was with a poorly differentiated mucinous adenocarcinoma.
An immunohistochemistry (IHC) analysis performed in April showed lack of MSH2 and EPCAM expression and the wild-type expression of KRAS-NRAS. To analyze the presence of MSI, a fluorescent PCR-based assay was performed (Promega MSI Analysis System). To this end, DNA samples were obtained from patient’s formalin-fixed paraffin-embedded tissue. Unfortunately, quality of the extracted DNA was insufficient for the assay. Although MMR proteins screening by IHC and microsatellite genotyping have similar effectiveness,Citation7,Citation31 MSI and IHC analysis are considered complementary to one another.Citation32 To characterize the lack of MMR protein expression, further genomic sequencing and deletion/duplication studies were conducted. The patient’s DNA sample was enriched for targeted regions using hybridization-based protocol, and sequencing was performed by using illumine technology (targeted regions were sequenced with ≥50× depth).
The analysis of genomic and deletion/duplication studies revealed a pathogenic heterozygous deletion involving both the EPCAM and MSH2 genes, which are located on the short (p) arm of chromosome 2 at position 21. All of the EPCAM’s nine exons were deleted (EX1–EX9del), whereas neighboring gene MSH2 had a deletion comprising exons 1–6 (EX1– EX6del). Since deletion of both genes is known to cause LS, the mutations were considered pathogenic. Other related genes, such as MLH1, MSH6, and PMS2, were screened; however, the results were negative. Likewise, no mutation was found in KRAS (KRAS/BRAF Mutation Analysis Panel Kit for Real-Time PCR, exons 2, 3, and 4 of KRAS and V600E of BRAF; EntroGen; Woodland Hills, CA, USA) or NRAS (NRAS Mutation Detection Kit, EntroGen). The patient received adjuvant treatment with 12 cycles of FOL-FOX (leucovorin, fluorouracil [5FU], and oxaliplatin) until November 29, 2015. After finishing the cycles of FOLFOX, the patient exhibited a reduction in CEA levels (from 237 in March 2015 to 27.9 in March 2016, not shown). However, a more detail analysis using PET/CT Citation18F-FDG scan revealed tumor lesions at two different locations: the intercostal nodule at the level of the 12th right rib () and a retrocrural adenopathy at the level of the 11th right dorsal vertebra (). The first PET/CT Citation18F-FDG scan after the 12 cycles of FOLFOX, conducted in April 2016, showed that the tumor mass at the intercostal nodule had reached 24 mm () and revealed a retrocrural adenopathy of 13 mm at the level of the 11th right dorsal vertebra ().
Figure 1 PET/CT Citation18F-FDG scan of tumor mass at the intercostal nodule at the level of the 12th right rib.
Abbreviations: PET/CT, positron emission tomography-computed tomography; SUV, standardized uptake value.
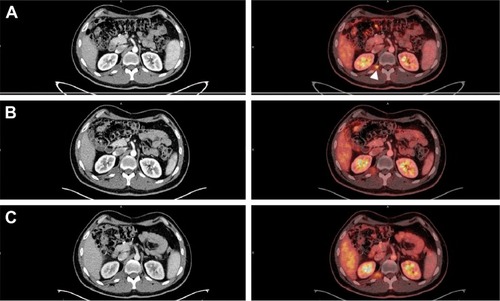
Figure 2 PET/CT Citation18F-FDG scan of the retrocrural adenopathy at the level of the 11th right dorsal vertebra.
Abbreviations: PET/CT, positron emission tomography-computed tomography; SUV, standardized uptake value.
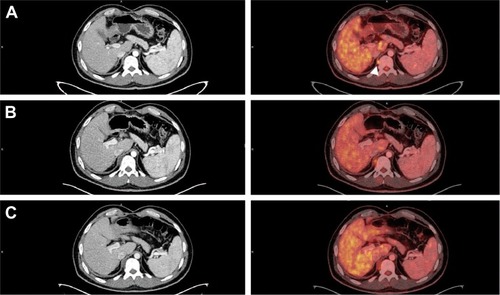
The biopsy confirmed that this new lesion corresponded to a metastatic mucinous adenocarcinoma. To treat these painful intercostal lesions, a new treatment scheme to block PD-1 with pembrolizumab (200 mg every 21 days) was implemented on June 23, 2016. After four cycles, the CEA levels were normalized (not shown), and the patient’s symptoms improved. We observed a slight size increase of the retrocrural adenopathy (at the 11th right dorsal vertebra) from 13 to 16 mm and the intercostal nodule (at the level of the 12th right rib) from 24 to 26 mm. However, both lesions showed less metabolic activity according to the PET/CT Standardized Uptake Value (SUV) (SUV[max] values of 4 and 3.2 instead of the previous 5.2 and 5.3, respectively; and ). These promising results led to six more cycles. In December, a new medical evaluation showed that the hypodense retrocrural nodule at the 11th right dorsal vertebra increased in size from 16 to 22 mm. Despite this, the metabolic activity from the previous SUV(max) value decreased from 4 to 3.5 (). Similarly, the intercostal nodule at the level of the 12th right rib experimented a slight increase from 26 to 28 mm; however, its metabolic activity rose from an SUV(max) value of 3.2 to 4.1 (). The patient followed two more cycles of pembrolizumab until February 2017, thus completing 12 cycles. In April 2017, he underwent palliative radiation and systemic therapy with FOLFIRI (Leucovorin Calcium, Fluorouracil and Irinotecan Hydrochloride) and Avastin, which continues until today.
Discussion
In this report, we expose the case of a patient with LS, harboring a deletion of all exons of EPCAM gene, which compromise the exons 1–6 from the neighbor MSH2. Deletion of EPCAM is present only in a subgroup of LS patients; however, this inactivation usually drives epigenetic silencing of MSH2 rather than deletion of this gene.Citation33 The EPCAM–MSH2 inactivation favors the appearance of other malignancies such as endometrial cancer.Citation34 Rossi et al showed that in Latin America, pathogenic variants of MSH2 are frequent, especially in Argentina.Citation14 Usually, these pathogenic variants are due to single nucleotide changes that produce frameshifts, missense and nonsense mutations. The most affected regions in MSH2 are exons 3 (17%) and 7 (15%), whereas our patient shows a large deletion compromising exons 1–6. Large deletions in EPCAM – affecting the 3′ region – are frequent, although, its prevalence in the Latin American population is relatively low.Citation14 Interestingly, our patient presented a large deletion compromising the function of both EPCAM and MSH2 genes. This mutation abolished the expression and function of MSH2 protein leading to the development of LS.
Recently, the therapeutic potential of antibodies to target relevant immunologic checkpoints in mismatch repair-deficient colon cancer patients has been scrutinized.Citation26–Citation28 Le et alCitation29 evaluated the clinical response to pembrolizumab in 41 patients with progressive metastatic carcinoma. The study compared between colorectal cancer patients with mismatch repair-deficient tumors (cohort A), patients with mismatch repair-proficient colorectal adenocarcinomas or microsatellite-stable cancers (cohort B), and patients with mismatch repair-deficient non-colon cancers (cohort C). Patients with mismatch repair-deficient cancers were more responsive to PD-1 blockade than those without mismatch repair-deficient cancers. In fact, 40% of the patients from cohort A had an immune-related objective response rate (95% CI, 12–74) according to the RECIST criteria. Seventy-one percent of cohort C patients had an immune-related objective response rate (95% CI, 29–96) and responded faster than patients from cohort A (12 weeks vs 28 weeks). On the contrary, patients from cohort B failed to show an immune-related objective response (95% CI, 0–19) as defined by RECIST. Moreover, the study showed that pembrolizumab treatment decreased the levels of CEA in patients with mismatch repair-deficient colorectal cancers, which was not the case for patients without mismatch repair-deficiency.Citation29 Our results support this observation as after four cycles with pembrolizumab the CEA levels in our patient were normalized.
A more recent study showed that a female patient with sporadic mismatch repair-deficient mCRC and bulky abdominal disease treated with pembrolizumab had a remarkable response.Citation28 Tumors with MMR deficiency have an elevated mutational load and, as a consequence, significant numbers of tumor-infiltrating lymphocytes in the tumor microenvironment.Citation26 Thus, PD-1 expression on lymphocytes and PD-L1 on tumor cells and the numbers of mutation-associated neoantigens are high in comparison with microsatellite-stable tumors.Citation29,Citation35
In this report, we describe the case of an LS-colorectal cancer patient, with mutations in EPCAM and MSH2. The patient was treated with pembrolizumab according to the molecular status of his cancer. This immunotherapy scheme delayed the second-line treatment with FOLFIRI and Avastin for at least 8 months, demonstrating to be an important therapeutic option. Although, the responsiveness of LS-colorectal cancer patients to pembrolizumab has been well documented, in South American countries it is not a standard procedure. Usually after diagnosis, LS patients follow a standard mCRC chemotherapy scheme as immunotherapy is expensive and not covered by public health insurances. Our results highlight the importance for South American countries to include LS-colorectal cancer patients in immunotherapy treatment programs. Especially, considering that a therapeutic scheme with pembrolizumab is less toxic than chemotherapy and has the potential to provide a durable response. The anti-PD-1 response of mismatch repair-deficient cancers is not restricted to LS colorectal cancers.Citation27 Therefore, the identification of MMR-deficient cancers will improve diagnostic and treatment strategies.
Author contributions
PS designed the study. CS-R and PS planned and wrote the paper. PS, SP, and MM treated the patient and RF performed the PET/CT Citation18F-FDG scans. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We owe thanks to the patient and his family and to the Instituto Oncológico Fundación Arturo López Pérez for supporting the publication of this work. Present address for Cristian Sozaried is at Escuela de Bioquímica, Facultad de Ciencia, Universidad San Sebastián and Instituto Oncológico Fundación Arturo López Pérez, Santiago, Región Metropolitana, Chile.
Disclosure
The authors report no conflicts of interest in this work.
References
- IonovYPeinadoMAMalkhosyanSShibataDPeruchoMUbiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesisNature199336364295585618505985
- ThibodeauSNBrenGSchaidDMicrosatellite instability in cancer of the proximal colonScience199326051098168198484122
- CarethersJMStoffelEMLynch syndrome and Lynch syndrome mimics: the growing complex landscape of hereditary colon cancerWorld J Gastroenterol201521319253926126309352
- CarethersJMDifferentiating Lynch-like from Lynch syndromeGastroenterology2014146360260424468183
- StoffelEMKastrinosFFamilial colorectal cancer, beyond Lynch syndromeClin Gastroenterol Hepatol20141271059106823962553
- GradyWMCarethersJMGenomic and epigenetic instability in colorectal cancer pathogenesisGastroenterology200813541079109918773902
- HampelHFrankelWLMartinEScreening for the Lynch syndrome (hereditary nonpolyposis colorectal cancer)N Engl J Med2005352181851186015872200
- WellsKWisePEHereditary colorectal cancer syndromesSurg Clin North Am201797360562528501250
- KheirelseidEAMillerNChangKHMismatch repair protein expression in colorectal cancerJ Gastrointest Oncol20134439740824294512
- DudleyJCLinMTLeDTEshlemanJRMicrosatellite instability as a biomarker for PD-1 blockadeClin Cancer Res201622481382026880610
- JenkinsMABagliettoLDowtyJGCancer risks for mismatch repair gene mutation carriers: a population-based early onset case-family studyClin Gastroenterol Hepatol20064448949816616355
- BolandCRKoiMChangDKCarethersJMThe biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedsideFam Cancer200871415217636426
- HampelHFrankelWPanescuJScreening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patientsCancer Res200666157810781716885385
- RossiBMPalmeroEILópez-KostnerFA survey of the clinicopathological and molecular characteristics of patients with suspected Lynch syndrome in Latin AmericaBMC Cancer201717162328874130
- StoffelEMHeritable gastrointestinal cancer syndromesGastroenterol Clin North Am201645350952727546846
- KobayashiHOhnoSSasakiYMatsuuraMHereditary breast and ovarian cancer susceptibility genes (review)Oncol Rep20133031019102923779253
- JärvinenHJAarnioMMustonenHControlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancerGastroenterology2000118582983410784581
- JiricnyJReplication errors: cha(lle)nging the genomeEMBO J19981722642764369822589
- HeinemannVvon WeikersthalLFDeckerTFOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trialLancet Oncol201415101065107525088940
- CremoliniCLoupakisFFalconeAFOLFOXIRI and bevacizumab for metastatic colorectal cancerN Engl J Med20153723291292
- MahipalAGrotheyARole of biologics in first-line treatment of colorectal cancerJ Oncol Pract201612121219122827943689
- van CutsemECervantesAAdamRESMO consensus guidelines for the management of patients with metastatic colorectal cancerAnn Oncol20162781386142227380959
- HodiFSO’DaySJMcdermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- BrahmerJRPD-1-targeted immunotherapy: recent clinical findingsClin Adv Hematol Oncol2012101067467523187774
- JanakiramMPareekVChengHNarasimhuluDMZangXImmune checkpoint blockade in human cancer therapy: lung cancer and hematologic malignanciesImmunotherapy20168780981927349980
- LeeVMurphyALeDTDiazLAMismatch repair deficiency and response to immune checkpoint blockadeOncologist201621101200121127412392
- LeDTDurhamJNSmithKNMismatch repair deficiency predicts response of solid tumors to PD-1 blockadeScience2017357634940941328596308
- KielerMScheithauerWZielinskiCCChottAAl-MukhtarAPragerGWCase report: impressive response to pembrolizumab in a patient with mismatch-repair deficient metastasized colorectal cancer and bulky diseaseESMO Open201616e00008428255450
- LeDTUramJNWangHPD-1 blockade in tumors with mismatch-repair deficiencyN Engl J Med2015372262509252026028255
- LeeVLeDTEfficacy of PD-1 blockade in tumors with MMR deficiencyImmunotherapy2016811326643016
- BarnetsonRATenesaAFarringtonSMIdentification and survival of carriers of mutations in DNA mismatch-repair genes in colon cancerN Engl J Med2006354262751276316807412
- UmarABolandCRTerdimanJPRevised Bethesda Guidelines for hereditary nonpolyposis colorectal cancer (Lynch syndrome) and microsatellite instabilityJ Natl Cancer Inst200496426126814970275
- LigtenbergMJKuiperRPChanTLHeritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3′ exons of TACSTD1Nat Genet200941111211719098912
- KempersMJKuiperRPOckeloenCWRisk of colorectal and endometrial cancers in EPCAM deletion-positive Lynch syndrome: a cohort studyLancet Oncol2011121495521145788
- GatalicaZSnyderCManeyTProgrammed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer typeCancer Epidemiol Biomarkers Prev201423122965297025392179
