Abstract
Background
Colorectal cancer stem cells (CRC-SCs) contribute to the initiation and progression of colorectal cancer (CRC). However, the underlying mechanisms for the propagation of CRC-SCs have remained elusive.
Purpose
The objective of this study was to study the role of NFATC2 in maintenance of the stemness in CRC-SCs.
Method
The expression levels of mRNA and protein were determined by qRT-PCR and western-blot, respectively. CRC-SCs were isolated by spheroid formation assay and flowcytometry. The sphere-forming and self-renewal abilities of CRC-SCs were determined by spheroid formation assay. The tumorigenicity of CRC-SCs was determined by cell-derived xenograft model. Gene manipulation was performed by lentivirus-mediated delivery system.
Results
We first found that NFATC2 is upregulated in primary CRC-SCs. Overexpression of NFATC2 promotes self-renewal and the expression of stem cell markers of CRC-SCs. Conversely, knockdown of NFATC2 attenuates stem cell-like properties of CRC-SCs. Mechanistic analysis indicated that NFATC2 upregulates the expression of AJUBA, downregulates the phosphorylation level of YAP, and therefore activates the transcriptional activities of YAP and promotes the stemness of CRC-SCs.
Conclusion
Our findings demonstrate NFATC2 as an oncogene that can promote the stemness of CRC-SCs. This work suggests a novel therapeutic strategy against CRC caused by aberrant expression of NFATC2.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Colorectal cancer (CRC) is the leading cause of cancer death.Citation1 Although current therapeutic strategies have reduced mortality, limited options are available for recurrence and resistance.Citation2 Cancer stem cells (CSCs) are a small subset of cancer cells capable of self-renewal and differentiating.Citation3 CSCs are responsible for tumor initiation and progression, resistant to chemotherapy and radiation, and thus have been recognized as a central hypothesis for the failure of cancer treatment.Citation4 CSCs have been identified in almost all solid tumors including prostate, ovary, breast as well as colorectal cancers.Citation5–Citation8 Identification of oncogenes and signaling pathways sustaining the properties of CSCs is critical for the development of an effective therapeutic strategy against CSCs.Citation9,Citation10
NFATC2 is a member of the nuclear factor of activated T-cell (NFAT) family which has well documented roles in the immune system.Citation11 However, its functions in cancer initiation and progression remained elusive. It has been reported that NFATC2 negatively regulates the promoter of cyclin-dependent kinase 4Citation12 and NFATC2 knockout mouse exhibits altered cell cycle control,Citation13 indicating a major role of NFATC2 in regulating the cell cycle. NFATC2 activates the mdm2 oncogene independent of P53Citation14 and enhances the metastasis of breast cancer cell by the upregulation of cyclooxygenase-2 and prostaglandins.Citation15 In addition, NFATC2 upregulates the mRNA level of human telomerase reverse transcriptase in activated peripheral blood lymphocytes.Citation16 Moreover, forced activation of NFATC2 activates c-Myc,Citation17 an important gene for the maintenance of stem cells, which suggests the potential role of NFATC2 in CSCs. Further efforts are still needed to explore the biological function of NFATC2 in CSCs.
Hippo is a highly conserved signaling cascade that regulates organ size and cell fate.Citation18 The core kinase cascade of Hippo pathway includes mammalian STE20-like protein kinase (MST1/2) and large tumor suppressor homolog (LATS1/2). In active state, Hippo signaling phosphorylates transcriptional co-activator YAP and results in its β-transducin repeat-containing E3 ubiquitin protein ligase (β-TRCP)-dependent degradation.Citation19 YAP regulates the expression of genes involved in key cellular functions, such as cell cycle, apoptosis, as well as maintenance of stem cell pluripotency.Citation20 Thus, YAP inhibition limits the organ size. Hippo/YAP axis is an important target for colorectal cancer therapy, and several small molecules for targeting various molecular components of Hippo/YAP signaling pathway have been developed.Citation21
In this study, we reported that NFATC2 promotes the stemness of CRC-SCs through AJUBA-mediated YAP activation. Our work thus demonstrates that NFATC2 is a novel therapeutic target for colorectal cancer stem cells (CRC-SCs) and suggests that YAP inhibitor could be an effective therapeutic strategy against CRC caused by aberrant expression of NFATC2.
Materials and methods
Cell culture and transfection
Human cancer cell lines (HCT-116, HT-29) were purchased from the American Type Culture Collection. Lenti-X 293T cell line was purchased from Clontech Laboratories (Palo Alto, CA, USA). Cell lines were cultured in DMEM with added fetal bovine serum, glutamine, penicillin, and streptomycin. The absence of mycoplasma contamination was confirmed using Myco Alert (Lonza, Basel, Switzerland).
Clinical samples
The tumor specimens were obtained from CRC patients who underwent surgery at the Department of Surgery at Shanghai Jiao Tong University-Affiliated Sixth People’s Hospital East Campus. This study was approved by Institutional Reviewing Board of Shanghai Jiao Tong University-Affiliated Sixth People’s Hospital East Campus. Written informed consent was obtained from each patient.
In vivo xenograft
All animal experiments were approved by the Institutional Animal Care and Use Committee at Chongqing Medical University. The experimental procedures all complied with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals; all guidelines for the welfare of the animals were followed.Citation22 Female nude mice have been injected with CRC sphere-derived cells. Kinetic of tumor formation was evaluated every 5 days.
Plasmids, primers, short hairpin RNAs (shRNAs), and reagents
Full-length cDNA of NFATC2 were generated by reverse-transcription PCR and cloned into pCDH expression vector. For shRNA experiments, two independent shRNAs against specific genes were cloned into the PLKO.1 vector. Primers are listed in , shRNAs are listed in , and antibodies are listed in . All common reagents were purchased from Sigma-Aldrich Co., St Louis, MO, USA.
Lentiviral generation and infection
Lenti-X Packaging Single Shots (Clontech Laboratories Inc.) were used to create lentiviruses. The cells were infected with media with lentivirus particles. Stable cell line was selected by puromycin for 10 days. The efficiency of knockdown and overexpression was determined by immunoblot.
Spheroid formation assay
The primary cells were isolated from tumor tissues and maintained in StemPro hESC SFM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 8 ng mL−1 basic fibroblast growth factor (Sigma-Aldrich Co.) in ultra-low attachment six-well plates (Corning Incorporated, Corning, NY, USA). The cells were seeded at a density of 1×103 cells per well into ultra-low attachment six-well plates (Corning Incorporated). After 10–15 days of culture, the spheroids were observed under a phase-contract microscopy (Leica Microsystems, Wetzlar, Germany).
Western blot
The protein samples were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto immobilon-P membranes (EMD Millipore, Billerica, MA, USA) followed by incubation with the primary antibodies at 4°C overnight. After incubation with the relevant horseradish peroxidase-conjugated secondary antibody and Clarity™ Western ECL Substrate (Bio-Rad Laboratories Inc., Hercules, CA, USA), signals were visualized with the ChemiDoc MP system (BioRad). Full scans of the blots are shown in Supplementary materials.
Immunofluorescence analysis
The cells or spheroids were rinsed and fixed using 4% para-formaldehyde for 10 minutes at room temperature. Antigen retrieval was performed by incubation with the antigen retrieval buffer (100 mM Tris, 5% urea, pH 9.5) at 95°C for 10 minutes. Cells were then permeabilized with PBS containing 0.1 Triton X-100 for 15 minutes. After blocking with 1% bovine serum albumin and 22.52 mg/mL glycine in PBS+ 0.1% Tween 20 for 30 minutes, cells were incubated with the primary antibody at 4°C overnight and then with the secondary antibody conjugated to DyLight 488, Alexa 488 or 555 (Abcam; Cambridge, MA, USA) for 1 hour at room temperature. Cells were examined using a total internal reflection fluorescence (TIRF) microscopy (Leica Microsystems, Wetzlar, Germany). Leica Application Suite X software (Leica Microsystems) was used to obtain the image.
Quantitative real-time reverse-transcription PCR
Total RNA was isolated with an RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands). cDNA was synthesized by Super-Script II reverse transcriptase (Thermo Fisher Scientific). The cDNA products were then subjected to TaqMan gene expression assay using specific primers. Amplification data were collected by ABI Prism 7000HT Real-Time PCR system. The results were calculated using the comparative threshold cycle (Ct) method with GAPDH as a control.
Statistical analysis
Each experiment was repeated for at least three times. Data are expressed as the mean ± SD. The significance of the data between experimental groups was determined by Student’s t-test (unpaired, two-tailed), *P<0.05, **P<0.01, ***P<0.001.
Results
NFATC2 is upregulated in CRC-SCs
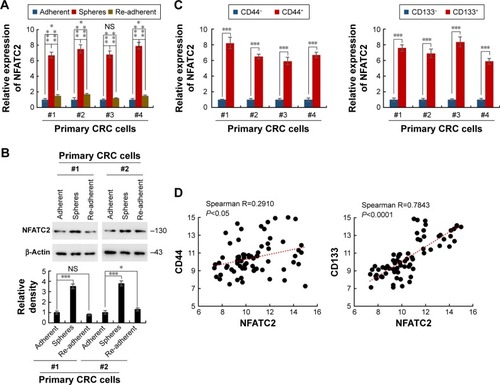
Sphere formation is recognized as the most prominent characteristic of CRC-SCs and can be used for in vitro study of CRC-SC function.Citation23 To investigate whether the expression level of NFATC2 is dysregulated in CRC-SCs, we first isolated the primary CRC spheres from tumor tissue of CRC patients by suspension culture.Citation24–Citation26 The mRNA and protein level of NFATC2 in the spheres and adherent cells were then analyzed by quantitative real-time polymerase chain reaction (qRT-PCR) and Western blot, respectively. As shown in , compared with adherent cells, NFATC2 expression was upregulated in CRC spheres. While, the expression level of NFATC2 was recovered to the level of origin when the spheres were reattached (). This result indicated that NFATC2 is upregulated in CRC-SCs. Next, as CD44 and CD133 are well-identified CRC-SC markers,Citation27,Citation28 to confirm this finding, we enriched CRC-SCs by flow cytometry sortingCitation24–Citation26 and examined the mRNA level of NFATC2 in CD44+ and CD133+ primary CRC cells and negative control cells. As shown in , the NFATC2 expression was upregulated in sorted CD44+ or CD133+ primary CRC cells, which confirmed that NFATC2 is upregulated in CRC-SCs. Furthermore, we examined the mRNA level of NFATC2, CD44, and CD133 in primary CRC spheres by qRT-PCR, and Spearman correlation analysis was subsequently performed. The results showed that the expression level of NFATC2 was positively correlated with the expression level of CD44 or CD133 in primary CRC spheres (). These data demonstrated that NFATC2 was upregulated in CRC-SCs.
Figure 1 NFATC2 is upregulated in CRC-SCs. (A) qRT-PCR analysis of NFATC2 in primary CRC spheres and re-adherent cells relative to adherent cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1, 2, 3, and 4). Spheres were obtained by suspension culture. (B) Western blot analysis of NFATC2 in primary CRC spheres and adherent and re-adherent cells. (C) qRT-PCR analysis of NFATC2 in sorted CD44+ (left) or CD133+ (right) primary CRC cells relative to negative cells. Primary CRC cells were isolated from the cancer tissues of colorectal cancer patients (No 1, 2, 3, and 4). CD44+ or CD133+ cells were obtained by flow cytometry. (D) The correlation between the transcription level of NFATC2 and CD44 (left) and CD133 (right) in pin primary CRC sphere-derived cells. The mRNA level of each gene was determined by qRT-PCR. Data were normalized to GAPDH as ∆CT and analyzed by Spearman’s correlation analysis. Data are represented as mean ± SD; *P<0.05, ***P<0.001; two-tailed Student’s t-test.
NFATC2 promotes CRC-SC properties of CRC cells
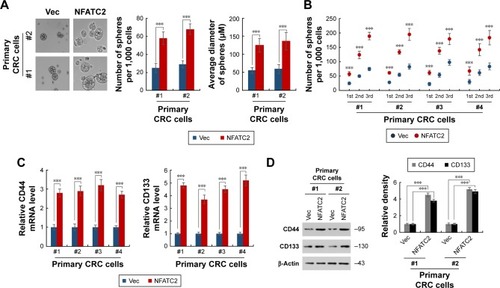
To further investigate the functional role of NFATC2 in CRC-SCs, we overexpressed NFATC2 in primary CRC cells by lentivirus delivery system (). As shown in , forced expression of NFATC2 in primary CRC cells significantly increased the number and size of the spheres, which indicated that NFATC2 promotes the sphere formation capacity of primary CRC cells. In addition, we examined the number of 1st, 2nd, and 3rd passaged spheres and found that NFATC2 promotes self-renewal capacity of primary CRC cells on serial passage (). Moreover, NFATC2 overexpression increased the expression of CSC-SC markers (CD44 and CD133) as indicated by qRT-PCR () and Western blot (). These results demonstrated that NFATC2 promotes CRC-SC properties of CRC cells.
Figure 2 Overexpression of NFATC2 promotes the stemness of CRC cells. (A, B) Sphere formation assay of NFATC2-overexpressing and control primary CRC cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). The 1st passaged spheres were obtained by suspension culture for 15 days and the number and average diameters of the spheres were counted (A). The number of 1st, 2nd, and 3rd passaged spheres isolated from the cancer tissues of CRC patients (No 1, 2, 3, and 4) was also counted (B). (C) qRT-PCR analysis of CD44 (left) and CD133 (right) in NFATC2-overexpressing and control primary CRC cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). NFATC2-overexpressing and control cells were generated by lentivirus delivery system. (D) Western blot analysis of CD44 and CD133 in NFATC2-overexpressing and control primary CRC cells. Data are represented as mean ± SD; ***P<0.001; two-tailed Student’s t-test.
NFATC2 is required for the maintenance of CRC-SCs
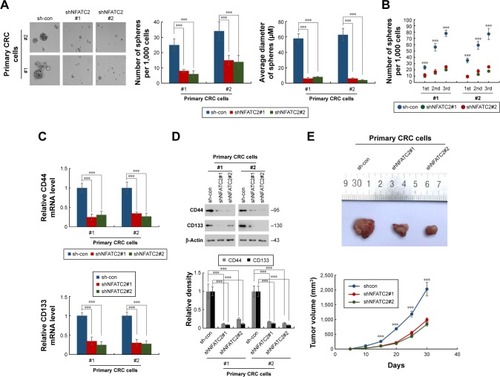
To further confirm the critical role of NFATC2 in the maintenance of CRC-SCs, we knocked down NFATC2 in primary CRC cells by two independent lentivirus-based shRNAs (). As shown in , knockdown of NFATC2 attenuates sphere-forming and self-renewal abilities of primary CRC cells. In addition, NFATC2-knockdown primary CRC cells exhibited decreased expression levels of CD44 and CD133 as identified by qRT-PCR and Western blot (). Furthermore, we examined the effect of NFATC depletion on tumorigenesis of primary CRC cells in vivo and found that NFATC depletion significantly decreased tumorigenesis of CRC cells in vivo. These results demonstrated that NFATC2 is required for the maintenance of the stemness of CRC-SCs.
Figure 3 Knockdown of NFATC2 inhibits the stemness of CRC cells. (A, B) Sphere formation assay of NFATC2-knockdown and control primary CRC cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). The 1st passaged spheres were obtained by suspension culture for 15 days and the number and average diameters of the spheres were counted (A). The number of 1st, 2nd, and 3rd passaged spheres was also counted (B). (C) qRT-PCR analysis of CD44 (top) and CD133 (bottom) in NFATC2-knockdown and control primary CRC cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). NFATC2-knockdown and control cells were generated by lentivirus delivery system. (D) Western blot analysis of CD44 and CD133 in NFATC2-knockdown and control primary CRC cells. (E) Tumorigenesis of NFATC2-knockdown and control primary CRC cells. Data are represented as mean ± SD; ***P<0.001; two-tailed Student’s t-test.
YAP activity is necessary for NFATC2-mediated CRC-SC properties
To reveal the underlying mechanism of NFATC2-mediated CRC-SC properties, NFATC2-overexpressing and knockdown HCT-116 and HT-29 stable cell lines () were generated and qRT-PCR array was performed to investigate the regulatory effect of NFATC2 on several signaling pathways which were recognized to be important for the maintenance of the properties of CRC-SCs, such as WntCitation29 and Hippo/YAPCitation18–Citation20 signaling pathway. As shown in , overexpression of NFATC2 significantly upregulated the expression levels of the target genes of Hippo/YAP signaling pathway (CTGF and Gli2)Citation18–Citation20 in both HCT-116 and HT-29 cells. Conversely, reduced mRNA expression levels of CTGF and Gli2 were observed in NFATC2-knockdown HCT-116 and HT-29 cells (). These results indicated that Hippo/YAP signaling might be the downstream effector of NFATC2.
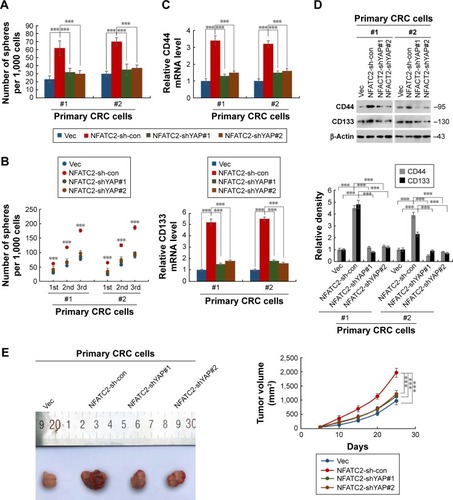
To confirm this finding, we next depleted YAP in NFATC2-overexpressing primary CRC cells () to investigate the role of YAP activity in NFATC2-induced CRC-SC properties. As shown in , knockdown of YAP abolished the stimulatory effect of NFATC2 on the sphere-forming and self-renewal abilities of CRC-SCs. In addition, YAP depletion recovered the increased expression levels of CD44 and CD133 in NFATC2-overexpressing primary CRC cells as indicated by qRT-PCR and Western blot (). Furthermore, YAP knockdown abolished the stimulatory effect of NFATC2 on tumorigenesis of primary CRC cells in vivo (), which indicated that YAP activity is critical for NFATC2 promoting the stemness of CRC-SCs.
Figure 4 YAP activity is necessary for NFATC2 for maintaining the stemness in CRC cells. (A, B) Sphere formation assay of YAP-knockdown NFATC2-overexpressing primary CRC cells and control cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). The 1st passaged spheres were obtained by suspension culture for 15 days and the number of the spheres were counted (A). The number of 1st, 2nd, and 3rd passaged spheres was also counted (B). (C) qRT-PCR analysis of CD44 (top) and CD133 (bottom) in YAP-knockdown NFATC2-overexpressing primary CRC cells and control cells. Primary CRC cells were isolated from the cancer tissues of CRC patients (No 1 and 2). YAP-knockdown NFATC2-overexpressing primary CRC cells and control cells were generated by lentivirus delivery system. (D) Western blot analysis of CD44 and CD133 in YAP-knockdown NFATC2-overexpressing primary CRC cells and control cells. (E) Tumorigenesis of YAP-knockdown NFATC2-overexpressing primary CRC cells and control cells. Data are represented as mean ± SD; ***P<0.001; two-tailed Student’s t-test.
The above results demonstrated that NFATC2 regulates Hippo/YAP signaling pathway in CRC cells and that YAP activity is critical for NFATC2-induced CRC-SC properties.
NFATC2 enhances YAP activity by the upregulation of AJUBA
To investigate the mechanism underlying the regulatory effect of NFATC2 on the activity of YAP, we first determined whether NFATC2 regulates YAP transcription. As shown in , there is no significant difference in YAP mRNA level between NFATC2-overexpressing cells and control cells (). While, as shown in , NFATC2 decreased the phosphorylation level of YAP in HCT-116 and HT-29 cells. In Hippo/YAP signaling pathway, a kinase cascade was involved in MST1/2, and LATS1/2 phosphorylates YAP and results in its β-TRCP-dependent degradation.Citation18–Citation20 Thus, this result indicated that NFATC2 might have affected the stabilization of YAP. To confirm this, we measured the effect of NFATC2 on the stabilization of endogenous YAP in HCT-116 cells following inhibition of new protein synthesis by cycloheximide (CHX). Indeed, as shown in , overexpression of NFATC2 stabilized the endogenous YAP in HCT-116 cells. These results thus demonstrated that NFATC2 activates YAP by promoting its stabilization and suggested that NFATC2 may target the upstream regulators of YAP.
Figure 5 NFATC2 activates Hippo/YAP signaling by upregulation of AJUBA. (A) Western blot analysis of phosphorylated YAP in NFATC2-overexpressing and control HCT-116 and HT-29 cells. (B) Western blot analysis of YAP in NFATC2-overexpressing and control HCT-116 and HT-29 cells pretreated with cycloheximide for the inhibition of new protein synthesis. (C) qRT-PCR analysis of AJUBA in NFATC2-overexpressing and control HCT-116 and HT-29 cells. (D) qRT-PCR analysis of CTGF and GLI2 in AJUBA-knockdown NFATC2-overexpressing and control HCT-116 and HT-29 cells. Data are represented as mean ± SD; ***P<0.001; two-tailed Student’s t-test.
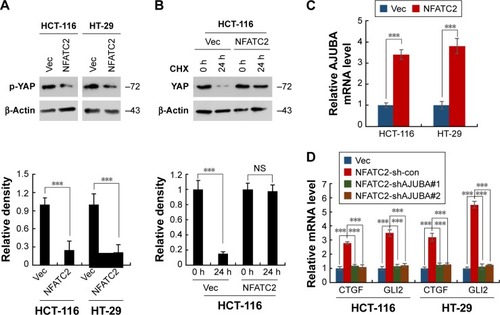
To further investigate the target of NFATC2 in Hippo/YAP signaling pathway, we performed qRT-PCR array to study the effect of NFATC2 on the transcription of key components in Hippo/YAP signaling pathway. As shown in , we found that NFATC2 significantly upregulates the mRNA level of AJUBA. As a fact that AJUBA activates YAP by inhibition of the kinase activity of LATS1/2,Citation30 we postulate that AJUBA mediates the stimulatory effect of NFATC2 on YAP activity. To confirm this, AJUBA was knocked down in NFATC2-overexpressing HCT-116 and HT-29 cells (), and the effect of NFATC2 on YAP activity was measured in these cells. As shown in , knockdown of AJUBA abolished the stimulatory effect of NFATC2 on the expression of CTGF and GLI2, indicating the critical role of AJUBA in NFATC2-induced upregulation of YAP activity. Taken together, the above results demonstrated that NFATC2 enhances YAP activity by the upregulation of AJUBA.
Discussion
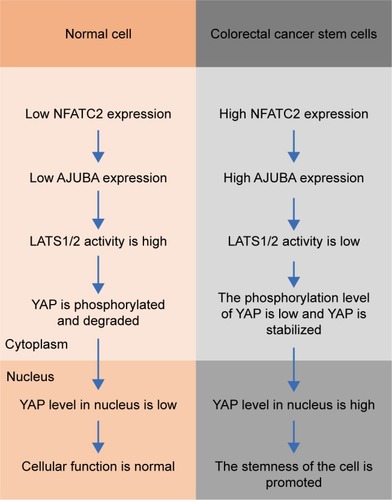
In this study, we reported that NFATC2 promotes the stemness of CRC-SCs through AJUBA-mediated activation of Hippo/YAP signaling ().
Figure 6 Schematic diagram of NFATC2 promoting the stemness of CRC-SCs. In colorectal cancer stem cells (right), the expression of NFATC2 is upregulated, compared to normal cells (left). Upregulated NFATC2 increases the expression level of AJUBA, and thus stabilizes YAP through AJUBA-mediated inhibition of LAST1/2, which phosphorylates YAP and promotes β-TRCP-dependent degradation of YAP. Stabilized YAP translocates into nucleus, activates the transcription of its target genes, and thereby promoting the stemness in colorectal cancer stem cells.
Accumulating evidence indicates that cancer initiation and progression are caused primarily by CSCs, a small subset of cancer cells, that shares stem-like properties through epithelial–mesenchymal transition (EMT).Citation31,Citation32 CSCs also contribute to recurrence, metastasis as well as chemo and radio-resistance in cancer.Citation1–Citation4 Thus, CSCs are the main target of cancer therapy. Although efforts toward understanding CSC biology in past decades identified a set of oncogenes and related signaling pathways sustaining “stemness,” still wide exploration is needed.
NFATC2 is the first identified member of the NFAT family, and its roles in the immune system are studied well.Citation11 Recent studies showed that there are potential functional roles of NFATC2 in the maintenance of CSCs. First, Zafari et al reported that the mRNA level of NFATC2 is upregulated in CRC tissues compared with normal tissue margins.Citation33 It has also been reported that NFATC2 regulates cyclin-dependent kinase 4 by directly binding its promoterCitation12 and mouse with NFATC2 knockout exhibits altered cell cycle control.Citation13 In addition, NFATC2 activates the mdm2 oncogeneCitation14 and enhances the metastasis of breast cancer cell by upregulation of cyclooxygenase-2 and prostaglandins.Citation15 Moreover, in activated peripheral blood lymphocytes, NFATC2 induces telomerase reverse transcriptase.Citation16 Furthermore, overexpression of NFATC2 activates c-Myc.Citation17 In our study, we first reported that NFATC2 is upregulated in CRC-SCs (). We subsequently showed that overexpression of NFATC2 promotes the stem cell-like properties of CRC-SCs (). Conversely, knockdown of NFATC2 attenuates these properties of CRC-SCs (), which demonstrated that NFATC2 is critical for the maintenance of CRC-SCs and is a novel therapeutic target of CRC-SCs.
Hippo/YAP axis is an important target for CRC therapy.Citation18,Citation19 Elevated YAP expression and the gene signature of high YAP activity have been found in most of the CRC patients and ApcMin/+ mouse model.Citation34,Citation35 YAP knockdown suppresses adenoma caused by loss of APC in mouse intestine.Citation36 YAP depletion inhibits the tumorigenicity of human CRC cell lines.Citation37 More importantly, YAP has been shown to be dispensable for normal tissue homeostasis, thus limiting the probability of side effects from YAP inhibition.Citation38 In this study, we found that NFATC2 upregulates the target genes of Hippo/YAP signaling pathway in CRC cells (). We then silenced YAP in NFATC2-overexpressing primary CRC cells and found that YAP depletion abolished the stimulatory effect of NFATC2 on self-renewal, expression of stem cell markers, and tumorigenicity of CRC-SCs (). Furthermore, we showed that AJUBA mediates the stimulatory effect of NFATC2 on YAP activity (). These results demonstrated that NFATC2 promotes the stemness of CRC-SCs mainly by suppression of Hippo signaling and thus suggested that YAP inhibitor could be an effective therapeutic strategy for human CRC caused by aberrant activation of NFATC2.
Conclusion
In summary, we demonstrate that NFATC2 promotes the stemness of CRC-SCs by AJUBA-mediated activation of Hippo/YAP signaling pathway. Our finding reveals that NFATC2 is a novel therapeutic target for CRC-SCs and suggests that YAP inhibitor could be an effective therapeutic strategy for human CRC caused by aberrant activation of NFATC2.
Acknowledgments
This work was supported by National Natural Science Foundation of China (81572080) and the Special Project for Social Livelihood and Technological Innovation of Chongqing (cstc2016shmszx130043).
Supplementary materials
Figure S1 Characterization of NFATC2-overexpressing primary CRC cells. Bottom panel: statistical analysis of the blots. Data are represented as mean ± SD; ***P<0.001; two-tailed Student’s t-test.
Figure S2 Characterization of NFATC2-knockdown primary CRC cells.
Abbreviations: CRC, colorectal cancer; NFATC2, nuclear factor of activated T-cells, cytoplasmic 2.
Figure S3 NFATC2 regulates Hippo/YAP signaling pathway. (A) Characterization of NFATC2-overexpressing HCT-116 and HT-29 cells. (B) Characterization of NFATC2-knockdown HCT-116 and HT-29 cells. (C) qRT-PCR analysis of CTGF and Gli2 in NFATC2-overexpressing HCT-116 and HT-29 cells. (D) qRT-PCR analysis of CTGF and Gli2 in NFATC2-knockdown HCT-116 and HT-29 cells. (E) Characterization of YAP-knockdown NFATC2-overexpressing CRC cells.
Abbreviations: CRC, colorectal cancer; NFATC2, nuclear factor of activated T-cells, cytoplasmic 2; qRT-PCR, quantitative real-time polymerase chain reaction.
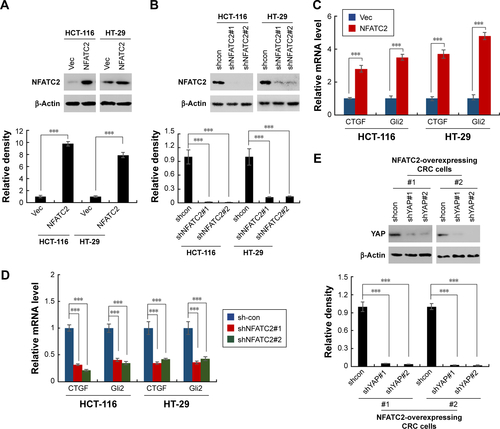
Figure S4 NFATC2 regulates Hippo/YAP signaling pathway through AJUBA. (A) qRT-PCR analysis of YAP in NFATC2-overexpressing HCT-116, HT-29 cells and their control cells. (B) Characterization of AJUBA-knockdown NFATC2-overexpressing HCT-116 and HT-29 cells.
Abbreviations: NFATC2, nuclear factor of activated T-cells, cytoplasmic 2; qRT-PCR, quantitative real-time polymerase chain reaction.
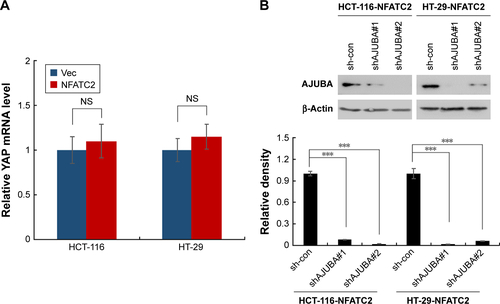
Table S1 Primer sequences used in this study
Table S2 shRNA sequences used in this study
Table S3 Antibodies used in this study
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDFedewaSAColorectal cancer statistics, 2017CA Cancer J Clin201767317719328248415
- FindlayVJWangCWatsonDKCampEREpithelial-to-mesenchymal transition and the cancer stem cell phenotype: insights from cancer biology with therapeutic implications for colorectal cancerCancer Gene Ther201421518118724787239
- ManciniRNotoAPisanuMEDe VitisCMaugeri-SaccàMCilibertoGMetabolic features of cancer stem cells: the emerging role of lipid metabolismOncogene201837182367237829445137
- DeanMFojoTBatesSTumour stem cells and drug resistanceNat Rev Cancer20055427528415803154
- WangZAShenMMRevisiting the concept of cancer stem cells in prostate cancerOncogene201130111261127121119602
- NgABarkerNOvary and fimbrial stem cells: biology, niche and cancer originsNat Rev Mol Cell Biol2015161062563826350076
- SmalleyMAshworthAStem cells and breast cancer: a field in transitNat Rev Cancer200331183284414668814
- VaiopoulosAGKostakisIDKoutsilierisMPapavassiliouAGColorectal cancer stem cellsStem Cells201230336337122232074
- ZhouBBZhangHDamelinMGelesKGGrindleyJCDirksPBTumour-initiating cells: challenges and opportunities for anticancer drug discoveryNat Rev Drug Discov200981080682319794444
- VisvaderJECells of origin in cancerNature2011469733031432221248838
- MacianFNFAT proteins: key regulators of T-cell development and functionNat Rev Immunol20055647248415928679
- MognolGPCarneiroFRRobbsBKFagetDVViolaJPCell cycle and apoptosis regulation by NFAT transcription factors: new roles for an old playerCell Death Dis20167e219927100893
- HodgeMRRangerAMCharles de la BrousseFHoeyTGrusbyMJGlimcherLHHyperproliferation and dysregulation of IL-4 expression in NF-ATp-deficient miceImmunity1996443974058612134
- ZhangXZhangZChengJTranscription factor NFAT1 activates the mdm2 oncogene independent of p53J Biol Chem201228736304683047622787160
- DuqueJFresnoMIñiguezMAExpression and function of the nuclear factor of activated T cells in colon carcinoma cells: involvement in the regulation of cyclooxygenase-2J Biol Chem2005280108686869315632146
- ChebelARouaultJPUrbanowiczITranscriptional activation of hTERT, the human telomerase reverse transcriptase, by nuclear factor of activated T cellsJ Biol Chem200928451357253573419843528
- MüllerMRRaoANFAT, immunity and cancer: a transcription factor comes of ageNat Rev Immunol201010964565620725108
- HongAWMengZGuanKLThe Hippo pathway in intestinal regeneration and diseaseNat Rev Gastroenterol Hepatol201613632433727147489
- PanDThe Hippo signaling pathway in development and cancerDev Cell201019449150520951342
- YuFXMengZPlouffeSWGuanKLHippo pathway regulation of gastrointestinal tissuesAnnu Rev Physiol20157720122725293527
- HongWGuanKLThe YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathwaySemin Cell Dev Biol201223778579322659496
- National Research Council, Guide for the Care and Use of Laboratory Animals8th edWashington, DCNational Acadamies Press2011
- ShaheenSAhmedMLorenziFNateriASSpheroid-formation (colonosphere) assay for in vitro assessment and expansion of stem cells in colon cancerStem Cell Rev201612449249927207017
- CammareriPLombardoYFrancipaneMGBonventreSTodaroMStassiGIsolation and culture of colon cancer stem cellsMethods Cell Biol20088631132418442654
- PrasetyantiPRZimberlinCDe SousaEMeloFMedemaJPIsolation and propagation of colon cancer stem cellsMethods Mol Biol2013103524725923959997
- DotseEBianYIsolation of colorectal cancer stem-like cellsCytotechnology201668460961925535115
- FangDDKimYJLeeCNExpansion of CD133(+) colon cancer cultures retaining stem cell properties to enable cancer stem cell target discoveryBr J Cancer201010281265127520332776
- DuLWangHHeLCD44 is of functional importance for colorectal cancer stem cellsClin Cancer Research2008142167516760
- ZhanTRindtorffNBoutrosMWnt signaling in cancerOncogene201736111461147327617575
- JagannathanRSchimizziGVZhangKAJUBA LIM proteins limit hippo activity in proliferating cells by sequestering the Hippo core kinase complex in the cytosolMol Cell Biol201636202526254227457617
- KresoADickJEEvolution of the cancer stem cell modelCell Stem Cell201414327529124607403
- CleversHThe cancer stem cell: premises, promises and challengesNat Med201117331331921386835
- ZafariVHashemzadehSHosseinpour FeiziMmRNA expression of nuclear factor of activated T-cells, cytoplasmic 2 (NFATc2) and peroxisome proliferator-activated receptor gamma (PPARG) transcription factors in colorectal carcinomaBosn J Basic Med Sci201717325526128504924
- HarveyKFZhangXThomasDMThe Hippo pathway and human cancerNat Rev Cancer201313424625723467301
- OverholtzerMZhangJSmolenGATransforming properties of YAP, a candidate oncogene on the chromosome 11q22 ampliconProc Natl Acad Sci U S A200610333124051241016894141
- CaiJMaitraAAndersRATaketoMMPanDβ-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesisGenes Dev201529141493150626193883
- GregorieffALiuYInanlouMRKhomchukYWranaJLYap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancerNature2015526757571571826503053
- BaiHZhangNXuYYes-associated protein regulates the hepatic response after bile duct ligationHepatology20125631097110722886419
