Abstract
Background
Yes-associated protein (YAP), a key player of the Hippo pathway, has been identified to have more and more important roles in tumorigenesis and may be an important biomarker for cancer therapy. YAP is important for bladder cancer cell migration, metastasis, and drug resistance; however, its function in bladder cancer stem cells remains unknown.
Purpose
The aim of this work was to examine the expression and role of YAP in bladder cancer stem cells.
Materials and methods
We identified that the expression level of YAP was significantly enriched in bladder cancer stem cells compared to noncancer stem cell population. Moreover, the effect of YAP on stem cell self-renewal was examined in bladder cancer cells by siRNA silencing approach. In addition, we showed that YAP is required for aldehyde dehydrogenase activity in bladder cancer cells.
Results
RNAseq analysis and quantitative real-time PCR results showed that silencing of YAP inhibited the expression of ALDH1A1 gene.
Conclusion
Collectively, our findings for the first time elucidated that YAP serves as a cancer stem cell regulator in bladder cancer, which provided a promising therapy strategy for patients with bladder cancer.
Keywords:
Introduction
Bladder cancer is the most common malignancy of the urinary system. Current standard treatment for muscle-invasive bladder cancer is surgery along with platinum-based chemotherapy.Citation1 However, still over 30% of bladder cancers managed by surgery recur and progress to locally invasive or metastatic stages.Citation2 Therefore, there is an unmet need for novel treatment to target bladder cancer. Cancer stem cells are rare population with stem cell characteristics, which play important roles in cancer chemoresistance, metastasis, and recurrence.Citation3 Understanding the mechanism under cancer stem cell regulation could lay the groundwork for design of effective treatment.
Mounting evidence has shown the existence of cancer stem cells in solid tumors. The common used markers, such as CD44, Bmi1, CD133, ALDH1, and ABCG2, are often used for isolation of cancer stem cells from cell lines.Citation4–Citation6 In bladder cancer cell lines, aldehyde dehydrogenase high (ALDHhigh) populations display resistance to cisplatin, increased colony-forming ability, migration, invasion, and ability to differentiate.Citation7 However, the regulation of ALDH is very limited.
Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) are the key players of the Hippo pathway, which are involved in organogenesis and tumorigenesis.Citation8 YAP has been increasingly demonstrated to play important roles in bladder cancer tumorigenesis, progression, and drug resistance. YAP activation protects bladder cancer cells from chemotherapy and radiation-induced DNA damage.Citation9 Moreover, YAP can interact with Nrf2 to maintain the chemoresistance in bladder cancer.Citation10 YAP-induced cell growth and migration in bladder cancer cells depends on transcriptional cofactor Mask2,Citation11 which can form a complex with YAP on target-gene promoters and is critical for YAP to activate transcription of target genes and tissue growth.Citation12
YAP/TAZ pathway plays an important role in the maintenance of CSC properties in various human cancers. For example, YAP can occupy the promoters of mammary stem cell signature gene to induce cancer stem cells in breast cancer.Citation13 In addition, YAP induced by SOX2 is essential for CSC characteristics in osteosarcoma and glioblastoma.Citation14 Taken together, this evidence indicates that YAP/TAZ plays critical roles in CSC maintenance and cancer progression. However, CSC-specific regulatory mechanisms of YAP/TAZ and Hippo pathway remain largely unknown.
In this study, we showed that YAP is upregulated in ALDHhigh populations of bladder cancer cell line. Silencing of YAP led to impaired self-renewal ability in bladder cancer cells. RNAseq results demonstrated that YAP can regulate the expression of ALDH1A1 in bladder cancer cells. In summary, we demonstrate that YAP is required for the stem cell property of bladder cancer stem cells via regulation of ALDH1A1.
Materials and methods
Cell culture and transfection
Human bladder cancer cell lines 5637 and SCaBER were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). The 5637 cells were maintained in DMEM (Invitrogen, Shanghai, China), supplemented with 10% fetal bovine serum (Invitrogen, Shanghai, China) in an incubator with 5% CO2 at 37°C. Subsequently, 20 mL of 10 mM scrambled control or YAP siRNAs were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and were transfected into 5637 using the Lipofectamine 2000 transfection reagent (Invitrogen, Shanghai, China) according to the manufacturer’s protocol. For YAP over-expression, cells were cultured to 40% confluence and then transfected with lentivirus-based human YAP expression constructs (addgene pGAMA-YAP).
FACS analysis and isolation
The ALDH enzymatic activity of 5637 cells was determined by ALDEFLUOR kit (Stem Cell Technologies, Shanghai, China), according to the manufacturer’s guidance. The data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA).
Sphere culture and colony formation assay
For sphere, 5637 and SCaBER cells were cultured in a stem cell growth medium containing DMEM/F12 basal media (Invitrogen, Shanghai, China), N2 and B27 supplements (Invitrogen, Shanghai, China), 20 ng/mL human recombinant epidermal growth factor, and 20 ng/mL basic fibroblastic growth factor in 24-well ultra-low attachment plates. Sphere numbers were counted 10 days after culture. For colony-forming assay, 1,000 cells were seeded into six-well culture plates and cultured for 14 days to allow colony formation. Colonies were then stained with 0.1% crystal violet for quantification.
Quantitative real-time PCR analysis (qPCR) and RNA sequencing
Total RNA was extracted from cells using the Trizol reagent (Invitrogen), and cDNA was synthesized from total RNA using the SuperScript III (Invitrogen) according to the manufacturer’s guidance. qPCR was performed using the Real-Time Quantitative PCR SYBR Green kit (Takara, Tokyo, Japan). Primer sequences used were β-actin forward 5′-AGGGGCCGGACTCGTCATACT-3′; β-actin reverse 5′-GGCGGCACCACCATGTACCCT-3′; YAP forward 5′-TAGCCC TGCGTAGCCAGTTA-3′; YAP reverse 5′-TCATGCTTAGTCCACTGTCTGT-3′; ALDH1A1 forward 5′-GCACGCCAGACTTACCTGTC-3′; ALDH1A1 reverse 5′-CCTCCTCAGTTGCAGGATTAAAG-3′. The relative expression of each gene was calculated using the 2ΔΔCT method relative to the expression levels of β-actin. For RNAseq library preparation, mRNA was then isolated, fragmented, and reverse-transcribed into the cDNA libraries with an mRNAseq preparation kit (Illumina, San Diego, CA, USA). Library quality was determined using Agilent Bioanalyzer 2100 (Agilent). The Illumina Hiseq 2000 platform was used for single-end sequencing of cDNA libraries. Alignment of reads was performed using Tophat2 with the hg19 build of the human genome. Transcript assembly and differential expression genes were identified by using Cufflinks software (Seattle, WA, USA). A heat map of differentially expressed genes involved in glutathione metabolism was generated using R.
Western blotting
Sorted cells were collected and nuclear protein was extracted using Nuclear Extraction Protocol (Thermo Fisher Scientific, Shanghai, China). Total amounts of total protein (30 µg) were separated using SDS-PAGE and transferred to a poly-vinylidene fluoride membrane. The membranes were blocked in 5% nonfat milk for 1 hour and incubated at 4°C overnight with primary antibodies: anti-CD133 (1:1,000, sc-101199, Santa Cruz), anti-histone H3 (1:5,000, Abcam, Shanghai, China). Signals were visualized after incubation with horseradish peroxidase-conjugated secondary antibody.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5.0 software. Data are represented as mean ± SD. Significant differences between groups were compared using two-tailed Student’s t-test. P<0.05 was considered statistically significant. *P<0.05, **P<0.01, ***P<0.001.
Results
Nucleus YAP is enriched in ALDHhigh population in bladder cancer cells
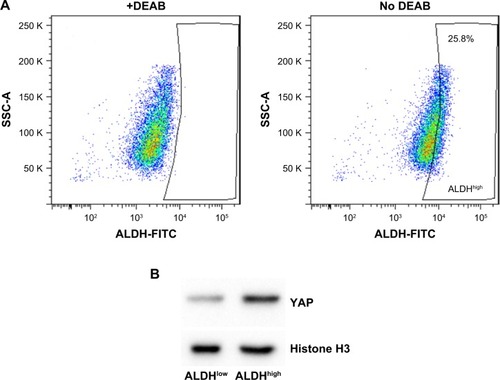
YAP has been previously reported to play essential roles in cancer stem cells’ regulation in various cancers.Citation13,Citation14 In 5637 bladder cancer cell line, the ALDHhigh cells display increased colony formation, migration, invasion, and ability to differentiate.Citation7 To investigate the possible role of YAP on bladder cancer stem cell regulation, we first isolated 5637 ALDHhigh subpopulation as assessed by the Aldefuor assay (). Indeed, the expression of YAP was nuclear-enriched in 5637 ALDHhigh subpopulation ().
Figure 1 YAP expression is elevated in ALDHhigh population.
Abbreviations: ALDHhigh, aldehyde dehydrogenase high; ALDHlow, aldehyde dehydrogenase low; YAP, Yes-associated protein.
YAP knockdown inhibited the colony-forming ability and self-renewal properties of bladder cancer stem cells
To explore the effect of YAP on the self-renewal ability in the bladder cancer stem cells, we transfected the 5637 cells with YAP siRNA (knockdown group) or scramble control (control group). First, we checked cells’ proliferation; however, we did not detect any difference (data not shown). Colony formation assay was then performed to determine the self-renewal capacity of 5637 cells. Our results showed a significant decrease in number of colonies in YAP-knockdown group compared to the control group ().
Figure 2 YAP is required for self-renewal capacity of bladder cancer cells.
Abbreviations: CFU, colony-forming unit; YAP, Yes-associated protein.
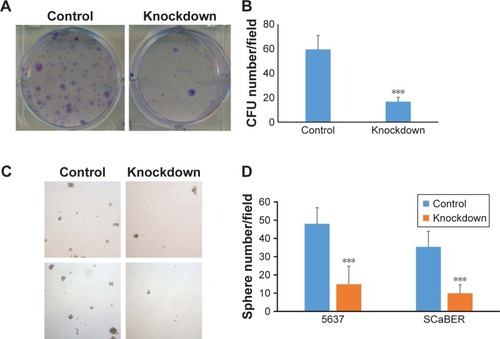
ALDHhigh 5637 and SCaBER cells were further isolated by FACS sorting and cultured in stem cell medium for sphere assay. YAP siRNA (knockdown group) or scramble control siRNA were added to the medium to determine the function of YAP on sphere formation of bladder cancer stem cells. As shown in , our results clearly demonstrated that YAP silencing caused decrease of sphere formation of ALDHhigh 5637 cells. Our data suggested that YAP functions in regulating the self-renewal capacity of the 5637 cells.
YAP is required for ALDH activity of bladder cancer
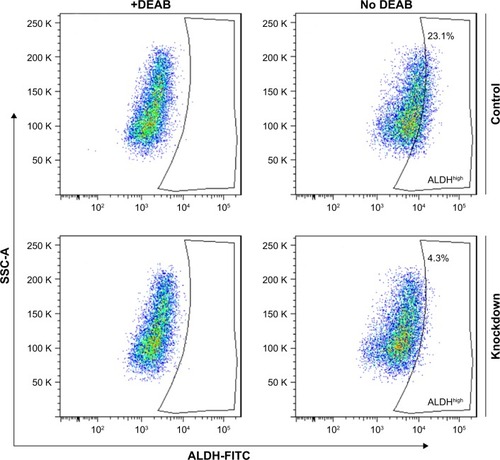
We next asked the question whether YAP depletion in 5637 cells could lead to inhibition of ALDHhigh subpopulation. To address that, we performed ALDH staining of both YAP knockdown and control 5637 cells. As shown in the flow cytometry results (), silencing of YAP significantly inhibited the ALDHhigh population of 5637 cells (23.1% vs 4.3%). This further supports that YAP is required for maintenance of cancer stem cell property in bladder cancer.
Figure 3 YAP is required for ALDH activity of bladder cancer cells.
Abbreviations: ALDH, aldehyde dehydrogenase; FITC, fluorescein isothiocyanate; YAP, Yes-associated protein.
YAP promotes self-renewal properties of bladder cancer stem cells
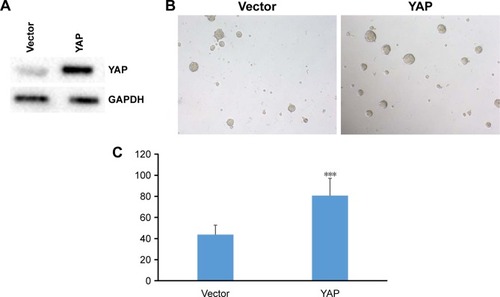
To further validate the role of YAP on stemness of 5637 cells, we performed YAP overexpression assay to test the ability of YAP on sphere formation of bladder cancer stem cells. As shown in , our results showed that YAP protein was upregulated in overexpression 5637 cells. In addition, our sphere assay showed that overexpression of YAP resulted in increase of sphere formation of 5637 cells (). Again, our data further proved that YAP functions in regulating the self-renewal capacity of the 5637 cells.
Figure 4 YAP promotes self-renewal properties of bladder cancer stem cells.
Abbreviation: YAP, Yes-associated protein.
YAP is required for ALDH1A1 expression of bladder cancer
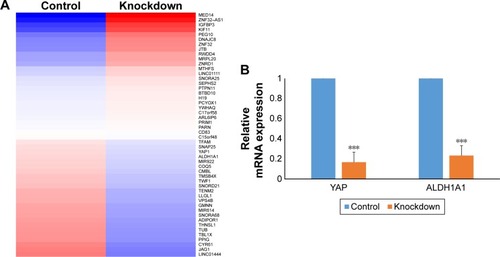
To further understand the downstream molecular events responsible for YAP regulation of bladder cancer stem cells, we extracted RNA from control and YAP knockdown 5637 cells and performed RNAseq. Our data showed that 343 transcripts were downregulated, and 143 transcripts were upregulated (fold change .2; P<0.01) ( and Table S1). Among the most downregulated genes, we found that both YAP and ALDH1A1 were both significantly inhibited in the knockdown group ( and Table S1). To verify our RNAseq results, we performed qPCR assay and found that depletion of YAP in 5637 cells significantly (P<0.001) reduced expression of ALDH1A1 (). Based on these results, it is concluded that YAP activity is required for ALDH1A1 expression.
Figure 5 ALDH1A1 expression depends on YAP in bladder cancer cells.
Abbreviation: YAP, Yes-associated protein.
Discussion
Cancer stem cells play key roles in drug resistance, metastasis, and recurrence, which are responsible for the majority of cancer mortality.Citation15 Hence, identifying cancer stem cells-related molecular mechanisms and characteristics is clinically important for developing better-targeted anticancer approach. The Hippo pathway regulates cell fate and stem cell differentiation during normal organogenesis and in the tumorigenesis.Citation8 Our study provides strong evidence that YAP is required for ALDH1A1 expression and the properties of cancer stem cells in bladder cancer, thus suggesting that YAP might be a promising target in the prevention and treatment of bladder cancer.
Hyperactivation or gene amplification of YAP and TAZ is commonly found in various types of cancer,Citation8 suggesting that YAP/TAZ is important for the development of neoplasia. In bladder cancer, previous study has shown that overexpression of YAP can promote cell growth and migration.Citation11 In addition, YAP/Nrf2 crosstalk is critical for bladder cancer chemoresistance.Citation10 In this study, we found that YAP is enriched in ALDHhigh population of 5637 cells, suggesting that YAP/TAZ pathway might serve as an oncogenic driver that confers cancer stem cell traits in bladder cancer.
Interestingly, we found that YAP might regulate the properties of cancer stem cells of bladder cancer via regulation of ALDH1A1 gene expression. However, whether YAP can directly regulate ALDH1A1 expression needs further validation. Using in silico analysis, we found that several potential YAP/TEAD binding sequences locate within the promoter regions of ALDH1A1 genes. It will be worthwhile to validate whether YAP can directly bind to these sequences using ChIP assay. In addition to ALDH1A1 regulation, the Hippo pathway has also been shown to regulate epithelial–mesenchymal transition and chemoresistance, suggesting alternate routes of YAP in regulating the properties of cancer stem cells. Indeed, our RNAseq data showed that many differentially expressed genes are involved in negative regulation of apoptotic process, suggesting that YAP might be important in antiapoptotic pathway. Despite its relevance in tumorigenesis, mutations in YAP are relatively limited. Combination of conventional therapy and inhibition of YAP/TAZ may be promising approaches to target cancer stem cells.
In summary, we found that YAP is upregulated in bladder cancer stem cells and is essential for self-renewal ability of bladder cancer stem cells. We delineated a YAP/ALDH1A1 pathway in regulating bladder cancer stem cells and have showed that these interactions might have important clinical implications.
Acknowledgments
We thank our colleagues in Department of Medical Oncology, The Second Affiliated Hospital of Fujian Medical University for their critique during manuscript preparation.
Disclosure
The authors report no conflicts of interest in this work.
References
- van KesselKEZuiverloonTCAlbertsARBoormansJLZwarthoffECTargeted therapies in bladder cancer: an overview of in vivo researchNat Rev Urol2015121268169426390971
- RouanneMLoriotYLebretTSoriaJCNovel therapeutic targets in advanced urothelial carcinomaCrit Rev Oncol Hematol20169810611526589398
- YangZLiCFanZSingle-cell sequencing reveals variants in ARID1A, GPRC5A and MLL2 driving self-renewal of human bladder cancer stem cellsEur Urol201771181227387124
- HatinaJParmarHSKripnerovaMHepburnAHeerRUrothelial carcinoma stem cells: current concepts, controversies, and methodsMethods Mol Biol2018165512113628889382
- FangDKitamuraHCancer stem cells and epithelial-mesenchymal transition in urothelial carcinoma: possible pathways and potential therapeutic approachesInt J Urol201825171728697535
- ChenDWuMLiYTargeting BMI1+ cancer stem cells overcomes chemoresistance and inhibits metastases in squamous cell carcinomaCell Stem Cell201720562163428285905
- FalsoMJBuchholzBAWhiteRWStem-like cells in bladder cancer cell lines with differential sensitivity to cisplatinAnticancer Res201232373373822399585
- MoroishiTHansenCGGuanKLThe emerging roles of YAP and TAZ in cancerNat Rev Cancer2015152737925592648
- CiamporceroEShenHRamakrishnanSYAP activation protects urothelial cell carcinoma from treatment-induced DNA damageOncogene201635121541155326119935
- CiamporceroEDagaMPizzimentiSCrosstalk between Nrf2 and YAP contributes to maintaining the antioxidant potential and chemoresistance in bladder cancerFree Radic Biol Med201811544745729248722
- DongLLinFWuWHuangWCaiZTranscriptional cofactor Mask2 is required for YAP-induced cell growth and migration in bladder cancer cellJ Cancer20167142132213827877230
- SidorCMBrainRThompsonBJMask proteins are cofactors of Yorkie/YAP in the Hippo pathwayCurr Biol201323322322823333315
- KimTYangSJHwangDA basal-like breast cancer-specific role for SRF-IL6 in YAP-induced cancer stemnessNat Commun201561018626671411
- Basu-RoyUBayinNSRattanakornKSox2 antagonizes the Hippo pathway to maintain stemness in cancer cellsNat Commun20156641125832504
- ShibueTWeinbergRAEMT, CSCs, and drug resistance: the mechanistic link and clinical implicationsNat Rev Clin Oncol2017141061162928397828
