Abstract
Background
Caveolin-2 (CAV2) is reported to have an important role in cancer. The following study investigated the expression and function of CAV2 in kidney cancer in vitro and in vivo.
Materials and methods
Real-time PCR, immunohistochemistry and Western blotting analysis were used to determine CAV2, epidermal growth factor receptor (EGFR), phosphatidylinositol 3-kinase (PI3K) and protein kinase B (Akt) in kidney cancer cell line OS-RC-2 and clinical specimens. The role of CAV2 in maintaining kidney cancer malignant phenotype was examined by wound healing assay, Matrigel invasion assays and mouse orthotopic xenograft model.
Results
Higher expression of CAV2 was found in renal cell carcinoma tissue compared to normal tissue. Furthermore, increased expression of CAV2 was associated with cancer progression. Also, silencing of CAV2 inhibited the proliferation, migration and invasion, as well as the expression of EGFR, PI3K and p-Akt in OS-RC-2 cells in vitro, and OS-RC-2 xenograft growth in vivo.
Conclusion
Our results revealed that CAV2 promotes the growth of renal cell carcinoma through EGFR/PI3K/Akt pathway.
Introduction
Renal cell carcinoma (RCC) is the most common type of renal malignant tumor. Currently, surgery is still considered as the main treatment approach for most types of RCC; even though its efficacy remains controversial.Citation1–Citation3 Drug resistance is common and represents a major cause of RCC death. RCC progression is usually accompanied with uncontrollable proliferation, distant metastasis and recurrence.Citation4–Citation7 However, the exact molecular mechanism of RCC is still unclear and needs to be further investigated.
Caveolin-2 (CAV2) is a member of the caveolae family which has an essential role in intracellular cell transport and signal transduction.Citation8 Higher expression of CAV2 has been associated with different types of cancer progression including lung, prostate, renal and breast cancer.Citation9–Citation12 In breast tumor, CAV2 expression has been strongly associated with high histological gradeCitation13 and poor prognosis.Citation14 Conflicting observations have been reported by Sagara et al, who found that CAV2 expression was suppressed in breast cancer tissues compared to normal tissues and that the reduced CAV1 was significantly associated with increasing tumor size.Citation15 In addition, a positive correlation between plasma CAV2 levels and progression of prostate cancerCitation16 and RCCCitation17 were also observed. Nevertheless, the exact role of CAV2 in RCC remains unexplored.
In this study, we found an abnormal expression of CAV2 in RCC tissues. In addition, we found that silencing of CAV2 inhibits tumor biological behavior in vitro and in vivo through the EGFR/PI3K/Akt pathway.
Materials and methods
Immunohistochemistry
The tissue chip, including 86 cancer tissues and 10 normal tissues were purchased from US Biomax (cat no KD2085, Xi An, China). The flow of immunostaining was performed by the streptavidin-peroxidase method.Citation18 The staining intensity was scored as 0, 1, 2 or 3, while percentage of stained cells was scored as 1 (<25%), 2 (26%–50%), 3 (51%–75%), or 4 (>75%). The final score was obtained by multiplying the intensity and percentage scores. The score below six scores were defined low expression. The score over six scores were defined high expression. The use of human samples was approved by the Ethics Committee of Chongqing Medical University.
RT-PCR (reverse transcription-polymerase chain reaction)
RT-PCR was done according to previously described method.Citation19 Briefly, total RNA was isolated from cancer cells using TRIzol reagent (cat no T9424, Sigma-Aldrich Co., Aldrich, MO, USA) according to the manufacturer’s protocol. All-in-One First-Strand cDNA Synthesis Kit was used to reverse transcribe the total RNA into cDNA (cat no 1708890, Bio-Rad Laboratories Inc., Hercules, CA, USA). Real-time PCR was performed with All-in-One™ qPCR mix (GeneCopoeia, Guanzhou, People’s Republic of China). The following primers were used: CAV2 (cat no HQP054857) and GAPDH (cat no HQP070342) purchased from GeneCopoeia. The experiments were performed in triplicate in the same reaction, and the results of the RT-PCR experiments were analyzed using the 2−ΔΔCt method.
Cell culture and reagents
Human RCC cells OS-RC-2 were acquired from Cell bank of Chinese Academy of Sciences (TCHu 40). The cells were maintained in RPMI-1640 medium supplemented with 10% FBS, 2 mM L-glutamine, 100 U/mL penicillin and streptomycin in a humidified atmosphere with 5% CO2 at 37°C. siRNA sequences were synthesized by GenePharma Co., Ltd (Shanghai, People’s Republic of China). The following sequences were targeted for CAV2. CAV2-1:5′-GCAAAUACGUAAUGUACAAGU-3′; CAV2-2:5′-GGAGAUUGGGAUACUGUAAUA-3′; and negative control siRNA: 5′-UUCUUCGAAGGUGUCACGU-3′.
CCK-8 assay
The cellular proliferation was determined by the CCK-8 assay (cat no CK04, Japan) according to the manufacturer’s instruction.Citation20,Citation21
Cell migration and invasion assays
The cell migration and invasion assays were performed in accordance with our previous studies.Citation22,Citation23 Briefly, OS-RC-2 cells were cultured in a six-well plate until reaching 80% confluency. The medium was replaced with serum-free medium. After the wounding, the distance between two wounds was measured at 0 and 72 hours. The invasion assays were performed as follows: the upper side was coated using Matrigel basement membrane matrix for 2 hours at 37°C. The OS-RC-2 cells were added into the top chamber, and then incubated for 48 hours; 6% paraformaldehyde was then used to fix the invasive cells. They were then stained in 0.5% crystal violet (Beyotime) and counted.
Western blot
Western blot analysis was performed as previously described.Citation24 Three independent experiments in a certain condition were subjected to Western blot analysis. The CAV2 (ab3417), EGFR (ab52894), PI3K (ab40776), Akt (ab38449) and GAPDH (ab8245) antibody were purchased from Abcam Inc. The antibody was dilution at 1:1,000.
Animals
BALB/c male nude mice, 6–8 weeks old, weighing 20–25 g, were obtained from Vital River Laboratories, China. All the animals were housed in an environment with temperature of 22°C ± 1°C, relative humidity of 50% ± 1% and a light/dark cycle of 12/12 hours. All animal studies (including the mice euthanasia procedure) were performed in compliance with the Accreditation of Laboratory Animal Care International (AAALAC) and Institutional Animal Care and Use Committee (IACUC) of Chongqing Medical University guidelines and approved by the IACUC of Chongqing Medical University. Mice were randomly divided in two groups: LV3-shCAV2-1 group (n=10) and LV3-NC group (n=10). Human RCC cells OS-RC-2 were infected with LV3-shCAV2-1 or LV3-NC and injected (5×106 cells per mouse in 200 μL) subcutaneously into the left armpit of nude mice. Then, 21 days later, animals were sacrificed under isoflurane anesthesia.
Statistical evaluation
All values were expressed as mean ± SEM. Statistical analysis was performed by Student’s t-test. A P-value of <0.05 was considered statistically significant.
Results
CAV2 expression was increased in RCC tissue
The ONCOMINE database was used to investigate differential genes expression.Citation25–Citation27 In this research, three independent studies from the ONCOMINE database were conducted to analyze the expression of CAV2 in RCC and normal kidney tissues. Three independent studies (Higgins Renal, Gumz Renal and Jones Renal) showed that the expression of CAV2 was higher in RCC compared to normal kidney tissues (fold changes were 5.716, 5.312, 8.918, 8.432, 2.618 and 4.156, respectively) (P=4.27E-12, 8.61E-19, 3.75E-4, 9.06E-4, 0.005 and 0.002, respectively) ().
Figure 1 CAV2 expression was elevated and associated with poor outcome in renal cell carcinoma.
Abbreviations: CAV2, Caveolin-2; OS, overall survival.
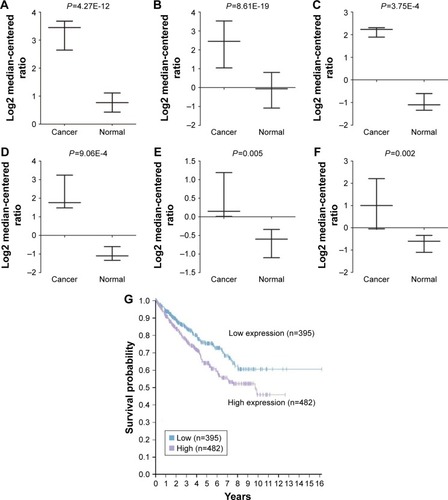
Furthermore, the evaluation of the CAV2 expression with OS was performed using the Human Protein Atlas online tool. Briefly, we found a significant correlation between high CAV2 and poor overall survival (OS) in patients with invasive RCC (P=3.44e-3; ).
The increased expression of CAV2 was associated with cancer progression
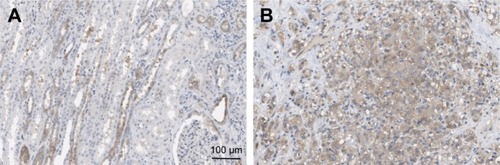
Next, we investigated the location and expression of CAV2 in RCC tissues and found that CAV2 was primary localized on the plasma membrane and cytoplasm (). In addition, the CAV2 expression was high staining in kidney carcinoma (). CAV2 in tubules cells was medium staining. However, CAV2 was not detected in glomeruli cells (). Furthermore, the CAV2 expression was correlated to tumor stage, and its expression was significantly higher in advanced stage (stage III/IV) compared to early stage tumor (stage I/II) (P<0.05; ). Moreover, the expression of CAV2 significantly correlated with the tumor grade (grades 2–3 vs 1, P<0.05; ). However, the associations between CAV2 expression and age were not significant (P>0.05; ).
Table 1 Association of CAV2 expression with clinicopathological characteristics in 86 patients of kidney cancer
Silencing of CAV2 inhibited proliferation, migration and invasion of OS-RC-2
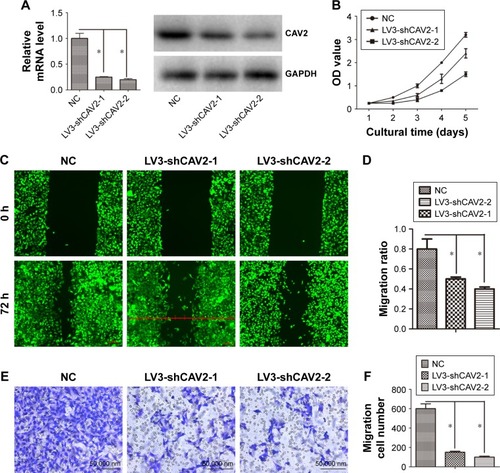
The expression of CAV2 was reduced in LV3-shCAV2-1 and LV3-shCAV2-2 infected OS-RC-2 cells compared with LV3-NC infected OS-RC-2 cells (). The cell proliferation, migration and invasion of OS-RC-2 cells infected with LV3-shCAV2-1 and LV3-shCAV2-2 decreased compared to cells infected with LV3-NC (P<0.05; ).
Figure 3 CAV2 regulates cellular proliferation, migration and invasion.
Abbreviation: CAV2, Caveolin-2.
CAV2 regulated EGFR/PI3K/Akt pathway
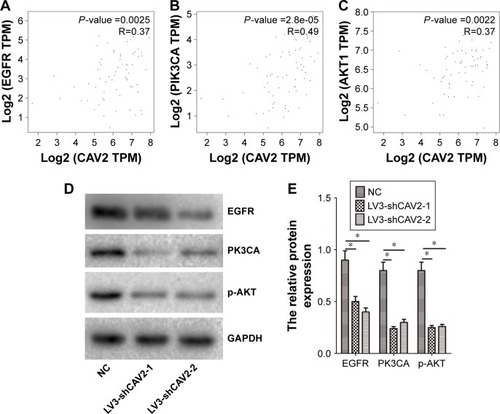
The EGFR/PI3K/Akt pathway has an important role in RCC. We further investigated the correlation between CAV2 and EGFR, PI3K and Akt using an online tool (http://gepia.cancer-pku.cn/detail.php). Our results showed that the expression of CAV2 in RCC was positively correlated with EGFR, PI3K and Akt (). In addition, we found that silencing of CAV2 reduces the expression of EGFR, PI3K and p-Akt in OS-RC-2 ().
Figure 4 CAV2 regulates the EGFR/PI3K/Akt signaling pathway.
Abbreviations: CAV2, Caveolin-2; EGFR, epidermal growth factor receptor; P13K, phosphatidylinositol 3-kinase; Akt, protein kinase B.
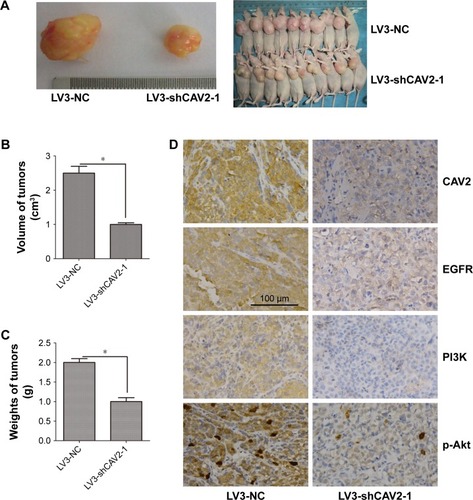
Silencing CAV2 inhibited the growth of OS-RC-2 cells in vivo
To investigate the role of CAV2 in vivo, OS-RC-2 cells infected with LV3-NC and LV3-shCAV2-1 were injected in nude mice. Briefly, the average tumor volume and weight were decreased in LV3-shCAV2-1 group compared to LV3-NC group (P<0.05) (). In addition, lower expression of EGFR, PI3K and p-Akt were found in tumors derived from LV3-shCAV2-1 compared to that in the LV3-NC group ().
Figure 5 CAV2 regulated tumorigenesis in nude mice model.
Abbreviations: CAV2, Caveolin-2; EGFR, epidermal growth factor receptor; P13K, phosphatidylinositol 3-kinase; Akt, protein kinase B.
Discussion
In this study, we found that CAV2 was abnormally expressed in RCC. The expression of CAV2 was related to the stage and grade of RCC, while high expression of CAV2 suggested a poor prognosis. We also found that CAV2 regulated the EGFR/PI3K/Akt signaling pathway.
Previous studies have found that CAV2 is associated with the occurrence and development of tumors. The expression of CAV2 has been observed in 5.9% of all breast cancer, while CAV2 expression has been reported as being strongly associated with high histological grade.Citation13 CAV2 is mainly expressed in breast cancers and is associated with poor prognosis.Citation14 However, another study found that CAV2 expression is suppressed in breast cancer tissues compared to normal tissues, and that the reduced CAV1 is significantly associated with increasing tumor size.Citation15 Another study has suggested that there is a correlation between plasma CAV2 levels and progression of prostate cancer.Citation16 CAV2 can promote tumor growth by supporting tumor-induced angiogenesis. Because of this function of CAV2 in tumor microenvironment, CAV2 is regarded as a potentially novel target for lung cancer therapy.Citation28 In esophageal squamous cell carcinoma, the increased expression of CAV2 correlates with a tumor progression and poor prognosis. So, CAV2, which has been shown to correlate with RCC,Citation17 is a potential biomarker for diagnosis of esophageal squamous cell carcinoma.Citation29 However, the expression and function of CAV2 is not known in RCC. Our results revealed that CAV2 had increased expression in RCC. The increased expression of CAV2 was associated with cancer progression. CAV2 high expression was significantly correlated with poor OS in all patients with invasive RCC. Silencing of CAV2 caused reduction in cell proliferation and growth with retarded entry into the S phase.Citation30 Our data revealed that silencing CAV2 inhibited the cellular proliferation, migration and invasion in RCC. Our results furthermore indicated that high expression of CAV2 promoted the progression of RCC.
The expression of EGFR correlates with prognosis in patients with clear cell RCC.Citation31 Suppression of the EGFR signaling pathway retards RCC progression.Citation32 The PI3K/AKT pathway is highly activated in RCC progression. This pathway is a promising drug target.Citation33–Citation37 In this research, we found that silencing CAV2 inhibited the expression of EGFR, PI3K and p-AKT in vitro and in vivo. We also found that the expression of CAV2 was positively correlated with EGFR, PI3K and AKT. Therefore, CAV2 may promote the malignant behavior through the EGFR/PI3K/AKT pathway in RCC. Inhibition of this pathway could serve as a promising target in RCC.
To sum up, our findings indicated that CAV2 played a role in promoting the growth of RCC. Inhibition of the EGFR/PI3K/AKT signaling pathway could be used as a potential approach for the treatment of RCC.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeissRHMetabolomics and metabolic reprogramming in kidney cancerSemin Nephrol201838217518229602399
- Pendón-Ruiz de MierMVAgüeraMLNavarroMDRodriguez-BenotAAljamaPPrevalence and survival of cancer after pancreas-kidney transplantationTransplant Proc201850266967229579884
- GeYWeygantNQuDAlternative splice variants of DCLK1 mark cancer stem cells, promote self-renewal and drug-resistance, and can be targeted to inhibit tumorigenesis in kidney cancerInt J Cancer Epub2018325
- ChangWHHorngHCYehCCRisks of female genital tract related cancers (gynecological cancers) or breast cancer in women with and without chronic kidney disease: a population-based cohort study in TaiwanMedicine20189712e015729561423
- SoriaFBeleniAID’AndreaDPseudoprogression and hyper-progression during immune checkpoint inhibitor therapy for urothelial and kidney cancerWorld J Urol Epub2018316
- KelseyRKidney cancer: ccrcc1–4 classification for prediction of relapseNat Rev Urol2018154205
- Torres da CostaESilvaVCostalongaECCoelhoFOCairesRABurdmannEAAssessment of kidney function in patients with cancerAdv Chronic Kidney Dis2018251495629499887
- HnaskoRLisantiMPThe biology of caveolae: lessons from caveolin knockout mice and implications for human diseaseMol Interv20033844546414993453
- FuSJJengCJMaCHUbiquitin ligase RNF138 promotes episodic Ataxia Type 2-associated aberrant degradation of human Cav2.1 (P/Q-Type) calcium channelsJ Neurosci20173792485250328167673
- ZhouXWangWZhangSCACNA1B (Cav2.2) overexpression and its association with clinicopathologic characteristics and unfavorable prognosis in non-small cell lung cancerDis Markers2017201761364016136401828127114
- TsouWLSoongBWPaulsonHLRodríguez-LebrónESplice isoform-specific suppression of the Cav2.1 variant underlying spinocerebellar ataxia type 6Neurobiol Dis201143353354221550405
- HarkinsABCahillALPowersJFTischlerASFoxAPDeletion of the synaptic protein interaction site of the N-type (CaV2.2) calcium channel inhibits secretion in mouse pheochromocytoma cellsProc Natl Acad Sci U S A200410142152191522415471993
- ElsheikhSEGreenARRakhaEACaveolin 1 and Caveolin 2 are associated with breast cancer basal-like and triple-negative immunophenotypeBr J Cancer200899232733418612310
- SavageKLeungSToddSKDistribution and significance of caveolin 2 expression in normal breast and invasive breast cancer: an immunofluorescence and immunohistochemical analysisBreast Cancer Res Treat2008110224525617912630
- SagaraYMimoriKYoshinagaKClinical significance of Caveolin-1, Caveolin-2 and HER2/neu mRNA expression in human breast cancerBr J Cancer200491595996515305200
- SugieSMukaiSYamasakiKKamibeppuTTsukinoHKamotoTSignificant association of Caveolin-1 and Caveolin-2 with prostate cancer progressionCancer Genomics Proteom2015126391396
- LiuXWangJSunGIdentification of key genes and pathways in renal cell carcinoma through expression profiling dataKidney Blood Press Res201540328829726043775
- ZhangXZhangYMiaoYZhouHJiangGWangETMEM17 depresses invasion and metastasis in lung cancer cells via ERK signaling pathwayOncotarget2017841706857069429050311
- MengFLiuHLiuSMaRThe clinical significance of HPIP and the associated prognosis in cervical cancerOncotarget2017841702627027029050277
- XuHFuSChenQThe function of oxytocin: a potential biomarker for prostate cancer diagnosis and promoter of prostate cancerOncotarget2017819312153122628415720
- JingsongHHongGYangJsiRNA-mediated suppression of collagen type iv alpha 2 (COL4A2) mRNA inhibits triple-negative breast cancer cell proliferation and migrationOncotarget2017822585259327906681
- WuHBiJPengYNuclear receptor NR4A1 is a tumor suppressor down-regulated in triple-negative breast cancerOncotarget2017833543645437728903348
- XueMQLiuJSangJFSuLYaoYZExpression characteristic of CXCR1 in different breast tissues and the relevance between its expression and efficacy of neo-adjuvant chemotherapy in breast cancerOncotarget2017830489304893728454081
- LiYJinLYeFIsoform expression patterns of EPHA10 protein mediate breast cancer progression by regulating the E-cadherin and β-catenin complexOncotarget2017818303443035628427223
- LeeALeeSHJungCKUse of the Ion AmpliSeq Cancer Hotspot Panel in clinical molecular pathology laboratories for analysis of solid tumours: with emphasis on validation with relevant single molecular pathology tests and the Oncomine Focus AssayPathol Res Pract2018214571371929615338
- YeLLiHZhangFLvTLiuHSongYExpression of KIF23 and its prognostic role in non-small cell lung cancer: analysis based on the data-mining of oncomineZhongguo Fei Ai Za Zhi2017201282282629277180
- XuBLiuNChenSQExpression of SRD5A1 and its prognostic role in prostate cancer: analysis based on the data-mining of ONCOMINEZhonghua Nan Ke Xue201622977177629071871
- LiuYJangSXieLSowaGHost deficiency in caveolin-2 inhibits lung carcinoma tumor growth by impairing tumor angiogenesisCancer Res201474226452646225269481
- AndoTIshiguroHKimuraMThe overexpression of caveolin-1 and caveolin-2 correlates with a poor prognosis and tumor progression in esophageal squamous cell carcinomaOncol Rep200718360160917671707
- LeeSKwonHJeongKPakYRegulation of cancer cell proliferation by caveolin-2 down-regulation and re-expressionInt J Oncol20113851395140221373752
- DorđevićGMatušan IlijašKHadžisejdićIMaričićAGrahovacBJonjićNEGFR protein overexpression correlates with chromosome 7 polysomy and poor prognostic parameters in clear cell renal cell carcinomaJ Biomed Sci2012194022475688
- LiangLLiLZengJInhibitory effect of silibinin on EGFR signal-induced renal cell carcinoma progression via suppression of the EGFR/MMP-9 signaling pathwayOncol Rep2012283999100522736024
- diJGaoKQuDYangJZhengJRap2B promotes angiogenesis via PI3K/AKT/VEGF signaling pathway in human renal cell carcinomaTumour Biol2017397 1010428317701653
- ZhaoZLiuHHouJTumor protein D52 (TPD52) inhibits growth and metastasis in renal cell carcinoma cells through the PI3K/Akt signaling pathwayOncol Res201725577377927983909
- ZhangHShengLTaoJDepletion of the triggering receptor expressed on myeloid cells 2 inhibits progression of renal cell carcinoma via regulating related protein expression and PTEN-PI3K/Akt pathwayInt J Oncol20164962498250627779645
- GuoHGermanPBaiSThe PI3K/AKT pathway and renal cell carcinomaJ Genet Genomics201542734335326233890
- SunZCaoBWuJProtease-activated receptor 2 enhances renal cell carcinoma cell invasion and migration via PI3K/AKT signaling pathwayExp Mol Pathol201598338238925773677