Abstract
The first-line treatment for metastatic esophageal squamous cell cancer (ESCC) is a platinum- or fluorouracil-based agent, followed by later treatment with taxanes or irinotecan. However, there is still no standard third-line treatment for patients with metastatic ESCC. We present a 62-year-old man initially diagnosed with locally advanced ESCC. After esophagectomy, the patient was administrated with six cycles of docetaxel and cisplatin combined with radiotherapy. After 8.0 months, computed tomography showed the left cervical lymph node metastasis. However, the metastatic lymph node was not significantly shrunk after locally palliative radiotherapy and the patient was intolerant of irinotecan as second-line systemic therapy. Then, the patient was rechallenged with six cycles of docetaxel combined with apatinib (an oral tyrosine kinase inhibitor to vascular endothelial growth factor receptor 2 [VEGFR2]), followed by single dose of apatinib as maintenance therapy. According to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard, partial response was achieved in this case after treating with docetaxel combined with apatinib. Now, the progression-free survival of this patient has been 7.5 months. After administrating with apatinib for 2 weeks, hypertension (grade III) was observed. Thus, the dose of apatinib was decreased from 850 to 500 mg and then the adverse effects were controllable and tolerable. In conclusion, apatinib with concurrent docetaxel provided potential efficacy as a salvage treatment for patients with metastatic ESCC. To our knowledge, this is the first case of ESCC who responded to apatinib combined with docetaxel.
Introduction
Esophageal cancer is the world’s eighth most common cancer and the sixth leading cause of cancer-related deaths.Citation1 Different from the situation in western countries, esophageal squamous cell carcinoma (ESCC) is still the dominant pathological type in China, which accounts for more than 95% of clinical cases.Citation2 The annual incidence of ESCC in China was about 260,000 and approximately half of the patients diagnosed with ESCC presented with metastatic diseases.Citation2 The first-line treatment for metastatic esophageal cancer is a platinum or fluorouracil-based agent,Citation3–Citation5 followed by later treatment with taxanesCitation6,Citation7 or irinotecan.Citation8,Citation9 No treatment strategy has been defined for patients who have been failed or intolerant to current standard therapies.
Angiogenesis is a critical process for cell growth, especially for the tumor growth.Citation10 The vascular epidermal growth factor (VEGF) could bind to vascular epidermal growth factor receptor (VEGFR) and activate the downstream pathway to stimulate the proliferation of vessel endothelium, then leading to the growth of tumor.Citation11 Previous molecular pathology studies have revealed that VEGF overexpression could be detected in about 44.43% of esophageal cancer and the estimated mortality risk was 1.82-fold greater in patients with high VEGF expression,Citation12,Citation13 which indicated that antiangiogenic agents may have a therapeutic effect on esophageal cancer.
Apatinib, a novel small molecule tyrosine kinase inhibitor (TKI), exerts its anti-tumor effects by specifically acting on VEGFR-2.Citation14 Phase II and III clinical trials suggested that apatinib could prolong overall survival (OS) of advanced gastric cancer patients who failed in the second-line treatment.Citation15,Citation16 In December 2014, China State Food and Drug Administration approved and launched apatinib as a treatment for patients with chemotherapy-refractory gastric cancer. Furthermore, many clinical studies have reported the satisfying efficacy of apatinib on various cancers including non-small-cell lung cancer,Citation17 breast cancer,Citation18,Citation19 and pancreatic cancer.Citation20 However, there is rare report to evaluate its efficacy and safety in patient with esophageal cancer. We herein report a unique case of ESCC receiving concurrent apatinib and docetaxel following failure of the second-line therapy. The progression-free survival (PFS) of this patient at least 7.5 months has been achieved now, demonstrating the potential of apatinib combined with chemotherapy in the treatment of ESCC. To our knowledge, this is the first case of ESCC who responded to apatinib combined with docetaxel.
Case report
A 62-year-old man suffered from dysphagia for almost 1 month was admitted to our hospital. Endoscopy biopsy suggested ESCC 28–35 cm away from incisors () and computed tomography (CT) indicated that there was a space-occupying lesion at the lower esophagus (). Physical examination did not show syndromes of hoarse voice and cough during drinking water, and no swelling superficial lymph node felt by touch. The patient had no history of hypertension, heart disease, diabetes, and kidney-related diseases.
Figure 1 Endoscopy biopsy (A) suggested esophageal squamous cell carcinoma; postoperative pathology (B) demonstrated moderately differentiated esophageal squamous cell carcinoma invading the full thickness of the esophageal wall.
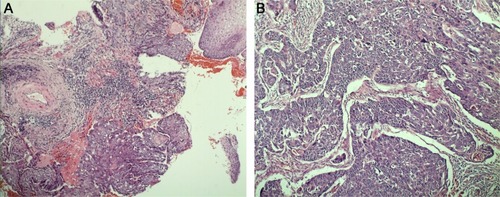
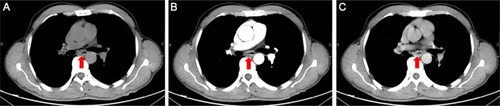
Figure 2 The initial thoracic CT indicated that the middle thickening esophageal wall was markedly enhanced. Plain scan (A); arterial phase (B); venous phase (C).
Abbreviation: CT, Computed tomography.
The esophagectomy was performed on September 2, 2016; postoperative pathological examination indicated moderately differentiated ESCC. The full thickness of the esophageal wall was invaded, with dimensions of 5 × 2 × 1.3 cm, and metastasis was observed in esophageal lymph nodes (3/7; ) but not observed in the resection margin. According to the eighth edition Staging Guidelines of American Joint Committee on Cancer, the surgical-pathological staging was T3N2M0, stage IIIB. Since September 2016, the patient received six cycles of chemotherapy of docetaxel (75 mg/m2, day 1) and cisplatin (70 mg/m2, day 1) combined with radiation therapy (total dose=50 Gy/25 fractions). In the follow-up after therapy, the gastroscopy and imaging examination showed no tumor recurrence. Until May 1, 2017, a lump could be touched in the patient’s left neck, shown as left cervical lymph node metastasis by CT (). Subsequently, locally radiotherapy (total dose=50 Gy/25 fractions) and three cycles of irinotecan (CPT-11, 250 mg/m2, 21 days as one cycle) monotherapy were performed; however, the patient suffered intolerable myelosuppression (grade IV) and severe gastrointestinal disturbances. Since CT revealed that the left cervical lymph node was not narrowed, the treatment was terminated (). After symptomatic treatment, the patient’s leucocytes returned to normal level, and the gastrointestinal adverse reactions disappeared.
Figure 3 Cervical CT showed no lymphadenopathy before surgery (A); CT showed a significantly enlarged lymph node in the left neck which prompted PD after first-line chemotherapy (B); CT revealed the left cervical lymph node was not reduced after radiotherapy and irinotecan treatment (C); CT examination showed the swollen lymph nodes significantly narrowed and the border was not clear, suggesting that the patient achieved PR (D).
Abbreviations: CT, Computed tomography; PD, disease progression; PR, partial response.
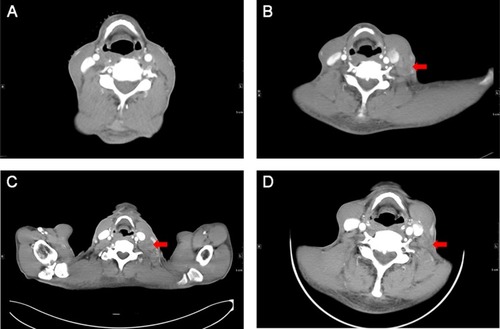
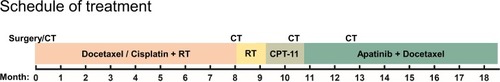
Since July 28, 2017, the patient received apatinib combined with six cycles of docetaxel (75 mg/m2, day 1, 21 days as one cycle) and then administrated apatinib as maintenance therapy. After treatment with apatinib for 1.4 months, CT examination showed cervical lymph node was decreased, without tumor recurrence or metastasis in other sites (). These results suggested that the patient achieved partial response (PR) according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 standard. There was no sign of progression in the patient’s conditions until we reported this case and now the PFS was 7.5 months (until March 10, 2018). The treatment schedule is given in .
Figure 4 The treatment schedule of the patient.
After apatinib was administrated for two weeks, hypertension (grade III) appeared, so the dose of apatinib was adjusted from 850 to 500 mg. During the apatinib treatment cycles, the patient developed mild-to-moderate hypertension and hand-foot syndromes. After treating with appropriate agents, no other anti-angiogenesis-associated adverse event (AE) was reported. Toxicity was evaluated and graded according to the NCI-CTC for Adverse Events, version 4.0.
The study was approved by the Medical Ethics Committee of the Affiliated Lianyungang Hospital of Xuzhou Medical University and written informed consent was obtained from the patient. The patient also provided his written informed consent for the case details and accompanying images to be published in this case study.
Discussion
To date, there has been no effective therapy as standard third-line therapy for improving the survival of ESCC patients due to its aggressive nature. To the best of our knowledge, this is the first case report on apatinib combined with docetaxel for ESCC treatment achieving PFS of 7.5 months. In addition, the patient tolerated well to the treatment, and reported satisfactory quality of life.
As early as 1971, professor Folkman suggested that tumor growth relied on the formation of tumor blood vessels, thus “anti-tumor angiogenesis” would be a promising strategy for treating tumors.Citation11 Based on previous researches, multiple antiangiogenic agents have been developed and studied in clinical trials. In the RAINBOW clinical trial, ramucirumab (a monoclonal antibody of VEGFR-2) combined with paclitaxel significantly increased OS compared with placebo combined with paclitaxel in advanced gastric or gastro-esophageal junction adenocarcinoma.Citation21 This agent was also advocated in treatment guidelines for esophageal adenocarcinoma.Citation22 Some clinical trials have demonstrated the effectiveness of bevacizumab as neoadjuvant therapy in ESCC.Citation23,Citation24 However, there are very few studies on anti-angiogenesis agents for metastatic ESCC.
Apatinib is a novel orally taken small molecular TKI, exerting its anti-angiogenesis function through highly and selectively competing with intracellular VEGFR-2’s adenosine triphosphate (ATP) binding sites. Thus, downstream signaling could be blocked to inhibit neovascularization in tumor tissue. The Phase II clinical trial of apatinib reported that median OS was increased to 4.83 months with apatinib 850 mg group, 4.27 months with apatinib 425 mg group, compared with 2.50 months with placebo group (P<0.001 and P=0.0017, respectively).Citation15 Similar results were confirmed by the Phase III study (median OS was 6.5 months in the apatinib 850 mg vs 4.7 months in the placebo group, P=0.0149; HR=0.709, 95% CI 0.537–0.937, P=0.0156).Citation16 The efficacy of apatinib on 62 advanced ESCC patients who failed the standard therapy was retrospectively analyzed,Citation25 among which, 15 received PR, while 31 achieved stable disease, with a manageable safety profile. These results demonstrated the preliminary efficacy and safety of apatinib for advanced ESCC.
Although antiangiogenic therapies show remarkable antitumor activity, their efficacy is limited as monotherapy. Hence, these agents have been integrated with conventional chemotherapy or radiotherapy to enhance antitumor activity.Citation26,Citation27 In our case, the patient received the docetaxel plus cisplatin regimen as first-line treatment and a PFS of 8.0 months was achieved. After the failure of standard chemotherapy and local radiation therapy of metastatic lesion, the patient still showed performance status score of 1, with strong willingness to continue the treatment. Thus, the docetaxel plus apatinib was administrated as salvage treatment strategy. Beyond our expectation, for this patient, the PFS of third-line treatment can be favorably comparable with the first-line treatment, indicating a potentially synergistic effect between apatinib and docetaxel chemotherapy. Moreover, recent research showed that the cytotoxicity of paclitaxel could be significantly enhanced by apatinib in vitro and in vivo by reversing chemotherapy-resistance through blocking the function of multiple ATP-binding cassette transporters.Citation28 Furthermore, apatinib can markedly increase the intracellular accumulation of conventional chemotherapy agents in side population cells sorted from K562 cells,Citation29 and cisplatin-resistant non-small-cell lung carcinoma A549 cell could be resensitized through suppressing extracellular signal-regulated kinase signaling pathway.Citation30 Currently, there are also multiple ongoing clinical trials investigating on apatinib for molecular targeted therapy in ESCC ().
Table 1 Selected ongoing trials with apatinib in metastatic ESCC
The common AEs of apatinib were hypertension and hand-foot syndrome.Citation31 According to the instruction of apatinib, we first prescribed the patient with apatinib of 850 mg daily. Then, grade III hypertension was observed, leading to dosage adjustment to 500 mg. The AEs were controllable and tolerable. The bioavailability of apatinib in ESCC patients was noteworthily higher than those in patients with gastric cancer, which might be attributed to the fact that most gastric cancer patient had a subtotal gastrectomy surgery history.Citation32
In conclusion, apatinib with concurrent docetaxel had potential efficacy for metastatic ESCC patients as a salvage treatment and the prospective clinical trials are ongoing ().
Acknowledgments
We thank the patients and all investigators involved in this study. The first authors of this manuscript are Li-Jun Liang, Yi-Xuan Wen, and You-You Xia.
Disclosure
The authors report no conflicts of interest in this work.
References
- TorreLABrayFSiegelRLCA: a cancer journal for clinicians20156528710825651787
- ChenWZhengRBaadePDCancer statistics in China, 2015CA Cancer J Clin201666211513226808342
- HayashiKAndoNWatanabeHPhase II evaluation of protracted infusion of cisplatin and 5-fluorouracil in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG) Trial (JCOG9407)Jpn J Clin Oncol200131941942311689594
- BleibergHConroyTPaillotBRandomised phase II study of cisplatin and 5-fluorouracil (5-FU) versus cisplatin alone in advanced squamous cell oesophageal cancerEur J Cancer1997338121612209301445
- HuangJZhouYZhangHA phase II study of biweekly paclitaxel and cisplatin chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma: ERCC1 expression predicts response to chemotherapyMed Oncol201330134323263828
- KatoKTaharaMHironakaSA phase II study of paclitaxel by weekly 1-h infusion for advanced or recurrent esophageal cancer in patients who had previously received platinum-based chemotherapyCancer Chemother Pharmacol20116761265127220703479
- MuroKHamaguchiTOhtsuAA phase II study of single-agent docetaxel in patients with metastatic esophageal cancerAnn Oncol200415695595915151954
- BurkartCBokemeyerCKlumpBPereiraPTeichmannRHartmannJTA phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancerAnticancer Res2007274C2845284817695458
- Mühr-WilkenshoffFHinkelbeinWOhnesorgeIA pilot study of irinotecan (CPT-11) as single-agent therapy in patients with locally advanced or metastatic esophageal carcinomaInt J Colorectal Dis200318433033412774248
- HanahanDWeinbergRAHallmarks of cancer: the next generationCell2011144564667421376230
- FerraraNAdamisAPTen years of anti-vascular endothelial growth factor therapyNat Rev Drug Discov201615638540326775688
- LuzCCFNogutiJAraújoLExpression of VEGF and Cox-2 in Patients with Esophageal Squamous Cell CarcinomaAsian Pac J Cancer Prev201819117117729373910
- ChenMCaiEHuangJYuPLiKPrognostic value of vascular endothelial growth factor expression in patients with esophageal cancer: a systematic review and meta-analysisCancer Epidemiol Biomarkers Prev20122171126113422564870
- TianSQuanHXieCYN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivoCancer Sci201110271374138021443688
- LiJQinSXuJApatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trialJ Clin Oncol201331263219322523918952
- LiJQinSXuJRandomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal JunctionJ Clin Oncol201634131448145426884585
- ZhangLShiMHuangCA phase II, multicenter, placebo-controlled trial of apatinib in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after two previous treatment regimensJournal of Clinical Oncology20123015_suppl7548
- HuXZhangJXuBMulticenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancerInt J Cancer201413581961196924604288
- HuXCaoJHuWMulticenter phase II study of apatinib in non-triple-negative metastatic breast cancerBMC Cancer20141482025376790
- LiCMLiuZCBaoYTSunXDWangLLExtraordinary response of metastatic pancreatic cancer to apatinib after failed chemotherapy: A case report and literature reviewWorld J Gastroenterol201723417478748829151702
- WilkeHMuroKRAINBOW Study GroupRamucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trialLancet Oncol201415111224123525240821
- AjaniJAD’AmicoTANational comprehensive cancer networkEsophageal and esophagogastric junction cancers, version 1. 2015J Natl Compr Canc Netw201513219422725691612
- IdelevichEKashtanHKleinYProspective phase II study of neoadjuvant therapy with cisplatin, 5-fluorouracil, and bevacizumab for locally advanced resectable esophageal cancerOnkologie2012357–842743122846974
- BendellJCMeluchAPeytonJA phase II trial of preoperative concurrent chemotherapy/radiation therapy plus bevacizumab/erlotinib in the treatment of localized esophageal cancerClin Adv Hematol Oncol201210743043722895283
- LiJWangLEfficacy and safety of apatinib treatment for advanced esophageal squamous cell carcinomaOnco Targets Ther2017103965396928860804
- ChengXXuZChenJApatinib to enhance chemosensitivity of gastric cancer to paciltaxel and 5-fluorouracilJ Clin Oncol20173515_supple15545
- ZhouFFengSZhangJAaJWangGCombined treatment of apatinib with docetaxel in non-small-cell lung cancer mice and its material basis of pharmacokineticsJournal of Clinical Oncology20173515_supple14069
- YjMLiangYJHuangHBApatinib (YN968D1) reverses multi-drug resistance by inhibiting the efflux function of multiple ATP-binding cassette transportersCancer Res201070207981799120876799
- TongXZWangFLiangSApatinib (YN968D1) enhances the efficacy of conventional chemotherapeutical drugs in side population cells and ABCB1-overexpressing leukemia cellsBiochem Pharmacol201283558659722212563
- LiuZLJinBJChengCGApatinib resensitizes cisplatin-resistant non-small cell lung carcinoma A549 cell through reversing multidrug resistance and suppressing ERK signaling pathwayEur Rev Med Pharmacol Sci201721235370537729243778
- LiJZhaoXChenLSafety and pharmacokinetics of novel selective vascular endothelial growth factor receptor-2 inhibitor YN968D1 in patients with advanced malignanciesBMC Cancer20101052920923544
- YuMGaoZDaiXPopulation Pharmacokinetic and Covariate Analysis of Apatinib, an Oral Tyrosine Kinase Inhibitor, in Healthy Volunteers and Patients with Solid TumorsClin Pharmacokinet2017561657627379402
