Abstract
Background
Retinoic acid induced 14 (RAI14), also known as NORPEG, is reported as being deregulated in non-small-cell lung cancer, together with having involvement in its cell proliferation as a super enhancer related gene.
Purpose
The objective of this study was to investigate the role of RAI14 in the progression and metastasis of gastric cancer and explore the associated mechanism.
Materials and methods
GEPIA database was used to analyze the expression of RAI14 in gastric cancer. MNK45 and AGS cells were transfected with siRNA-RAI14 to block the expression of RAI14. Cell Counting Kit 8 and colony formation assays were performed to measure cell proliferation. Cell migration and invasion capacities was examined by transwell assay. Apoptosis rate was detected using flow cytometry, and the protein levels of apoptosis-related proteins was determined using Western blot assay. Reverse-transcription PCR assay was used to detect the expressions of RAB31.
Results
Gene expression profiling interactive analysis revealed that RAI14 was substantially upregu-lated in gastric cancer and higher expression of RAI14 was associated with worse prognosis. We also observed that the knockdown of RAI14 by siRNA-RAI14 transfection suppressed growth capacity of MKN45 and AGS cells. Also, RAI14 knockdown inhibited migration and invasion of MKN45 and AGS cells in vitro. Moreover, RAI14 knockdown was observed to accelerate cell apoptosis via down-regulation of Bcl-2 and upregulation of Bax in MKN45 and AGS cells. Furthermore, downregulation of RAI14 inhibited the activation of Akt pathway, and activation of Akt by IGF-1 could restore the reduced proliferation induced by RAI14 knockdown. In addition, we found that RAI14 had a positive correlation with the RAB31 in gastric cancer by GEPIA reverse-transcription PCR and Western blot assays, and the reduced proliferation caused by RAI14 knockdown was restored by RAB31.
Conclusion
RAI14 knockdown inhibited proliferation, migration and invasion and promoted apoptosis by downregulating the Akt pathway in gastric cancer cells, and RAB31 might be a downstream target gene of RAI14, providing a novel sight into the molecular mechanism of RAI14 and a potential target for gastric cancer treatment.
Introduction
Gastric cancer is one of the major malignancies, causing many cancer-related deaths in the world, with approximately 951,000 new cases, leading to approximately 723,000 deaths each year.Citation1,Citation2 Although great progress has been made in the clinical treatment and diagnosis of gastric cancer in the past decade, the 5-year survival rate of patients following the radical operation is only between 30% and 50%.Citation3,Citation4 Accordingly, identification of the novel molecular target, which is able to repress the progression and metastasis of gastric cancer, is expected to facilitate the development of new cancer therapy strategies.
Retinoic acid induced 14 (RAI14), known as NORPEG as well, is an actin-binding protein initially identified in the liver.Citation5 Previous studies identify that RAI14 is expressed in a great deal of mammalian tissues or cells, but is primarily expressed in retina, placenta and testes and highly expressed in spermatozoa.Citation6–Citation9 RAI14 is observed to be a regulatory protein at the ectoplasmic specialization, in addition to being confirmed to be involved in maintaining mouse spermatid polarity and cell adhesion through the regulation of the F-actin dynamics.Citation9,Citation10 Recent studies highlight that RAI14 is a super enhancer (SE)-related gene that is upregulated in non-small-cell lung cancer (NSCLC) cell A549 and a portion of tumor tissues (43.66%, 32/71), and functions as a potential bio-marker for the patients with lung adenocarcinoma.Citation11 SE is a large cis-regulatory element enhancing the expression of crucial genes that could define cell identity, in addition to playing a pivotal role in the process of development and cancer.Citation12–Citation14 SEs are reported to be enriched at oncogenes in cancer cells, which are generated and activated through chromosomal rearrangements, focal amplification, and upregulation of transcription factors.Citation11,Citation14,Citation15 As confirmed by multiple research works, the upregulation of SE is involved in the tumor pathogenesis.Citation16 Besides that, SEs in cancer cells are more susceptible to perturbation as compared with the typical enhancer, which leads to a more pronounced impairment of the expression of SEs-related genes.Citation17–Citation19 As these studies suggest, it is speculated that RAI14 might be associated with the tumor progression, but there are few investigations dealing with this point, in particular in gastric cancer.
Herein, we demonstrate that RAI14 was upregulated in gastric cancer associated with the patient’s prognosis, and RAI14 knockdown by siRNA interference reduced proliferation and migration, promoted apoptosis through inhibiting the activation of Akt signaling pathway in gastric cancer.
Materials and methods
Cell culture and transfection
The human gastric cancer cell lines MKN45 and AGS were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, People’s Republic of China). Cells were cultured in DMEM (Hyclone, Thermo Fisher Scientific, Waltham, MA, USA) medium that contained 10% FBS (Gibco, Thermo Fisher Scientific) at 37°C with 5% CO2. Cells were treated with IGF (50 ng/mL, R&D Systems Inc., Minneapolis, MN, USA) for 24 hours to activate Akt signaling pathway, or treated with LY294002 (3 µM; MedChemExpress, Monmouth Junction, NJ, USA) for 24 hours to inhibit the phosphorylation of Akt.
The siRNA-RAI14 sequence was obtained from Oligobio (Beijing, People’s Republic of China). Cells were transfected with siRNA-RAI14 (RAI14 knockdown, RAI14-KD) or siRNA negative control (NC) using Lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific) in accordance with the manufacturer’s protocol. Cells without any treatment constituted the blank group. Cells were transfected with pcDNA3.1-RAI14 (RAI14 overexpression, RAI14-OV) to upregulate the expression of RAI14, and pcDNA3.1 was used as the NC by Lipofectamine 2000.
Western blot
Western blot assay was carried out for the purpose of examining the relative expression of RAI14 and other related proteins according to the protocol. RIPA Lysis Buffer (CWBIO, Shanghai, People’s Republic of China) was performed to lyse cells for protein extraction. The protein concentration was examined by BCA Protein Assay Kit (Beyotime, Jiangsu, People’s Republic of China) and 20 µg of protein of each sample was separated by 10% SDS-PAGE gel. Thereafter, proteins were transferred to polyvinylidene fluoride membrane, and incubated with the primary antibodies in blocking solution at 4°C overnight. Subsequent to that, the membrane was incubated with the secondary antibody for 1 hour. An enhanced chemiluminescence kit was performed for the signal development. The primary antibodies were as follows: anti-RAI14 (Proteintech Group Inc., Rosemont, IL, USA), anti-Bcl-2 (Abcam, Cambridge, UK), anti-Bax (Abcam), anti-Akt (Abcam), anti-p-Akt (Abcam), anti-Cyclin D1 (Abcam), and anti-GAPDH (Abcam). The secondary antibodies were obtained from Proteintech Group Inc.
Cell proliferation and colony formation assay
For CCK8 assay, approximately 1×103 cells per well were seeded into 96-well plates. Then 10 µL of CCK8 reagent (Solarbio Science & Technology, Beijing, People’s Republic of China) was added into each well, followed by incubating for 90 minutes at 37°C. The OD value of excitation light was measured every 24 hours using enzyme standard instrument with 450 nm. In regard to the colony formation assay, almost 500 cells were seeded into 10 cm dish containing 5 mL medium, and cultured at 37°C with 5% CO2. Following the formation of sufficiently large clones, cells were stained with 0.1% crystal violet. The image was recorded and the number of colonies were counted as well.
Cell migration assay
Transwell chamber was used to assess cell migration and invasion. For invasion assay, chambers coated with Matrigel were used for invasion assay. Cells were trypsinized and resuspended in serum-free culture medium, and almost 1×104 cells were transferred to the upper chamber, and complete medium was added to the lower chamber. Subsequent to incubation for 24 hours, the invasive cells were fixed with 4% paraformaldehyde, followed by staining with 0.1% crystal violet for 5 minutes. The invasive cells were imaged and counted under the microscope. With respect to the migration assay, a method similar to the invasion assay employed; other than that, Matrigel was absent and the number of cells was 5×103.
Cell apoptosis assay
Flow cytometry was carried out for the detection of the cell apoptosis using Annexin V-FITC-PI Apoptosis Detection Kit (4A Biotech Co., Beijing, People’s Republic of China). Cells were resuspended in 1× binding buffer at a density of 1–5×106 cells/mL. The 100 µL cell suspensions were incubated with 5 µL of Annexin V-FITC for 5 minutes in the dark, followed by the addition of 10 µL of PI and 400 µL of PBS. The samples were analyzed using FACSCalibur instrument and analyzed by BD FACSDiva software.
Quantitative Reverse-Transcription PCR (RT-qPCR)
Total RNA was extracted using Trizol (PuFei, Shanghai, People’s Republic of China) and reverse transcribed to cDNA by using the SuperRT cDNA Synthesis kit (CWBIO). The expression of mRNAs was detected using SYBR Master Mixture (Takara Bio Inc., Kusatsu, People’s Republic of China). Primers were as followed: RAI14 upstream: 5′-GTGGATGTGACAGCCCAAGA-3′, downstream: 5′-TTTCCCAGAGCTGTCGACAC-3′; RAB31 upstream: 5′-TTGACCACAACATCAGCCCT-3′, downstream: 5′-ACATCCCCTCCATGTGCATT-3′. Analysis of the relative expression of target gene was performed by the comparative Ct value.
Statistical analysis
The data were represented from three independent experiments and expressed as mean ± SD. The comparisons between the two groups were analyzed using Student’s t-test. SPSS 18.0 software was used for analyzing the data in the present study. P<0.05 was considered statistically significant.
Results
Expression of RAI14 is upregulated in gastric cancer and associated with the poor prognosis
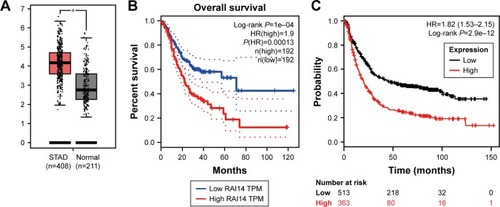
Gene expression profiling interactive analysis (GEPIA) is a website for a deep understanding of gene functions through excavation of the RNA sequencing data from TCGA and GTEx databases.Citation20 The expression level of RAI14 in gastric cancer tissue was substantially higher in comparison with that of the normal tissue by GEPIA, which suggested that RAI14 is upreg-ulated in gastric cancer (). Further prognostic analysis revealed that the overall survival (OS) of gastric cancer patients with higher RAI14 expression level was significantly worse as compared with that of the patient with lower expression level, indicating that RAI14 expression level had correlation with the prognosis of gastric cancer patients (P=0.0001, ). Survival analysis results from Kaplan–Meier Plotter (http://kmplot.com/analysis/index.php?p=service&cancer=gastric) shed light on the fact that patients with low expression of RAI14 had better prognosis in comparison with the patients with high expression (P=2.9e-12, ). Based on these results, RAI14 might be involved in gastric cancer and correlated with the prognosis of gastric cancer patients.
Figure 1 RAI14 downregulation is associated with prognosis of gastric cancer patients and reduces proliferation and viability of gastric cancer cells.
Abbreviations: GEPIA, gene expression profiling interactive analysis; RT-PCR, reverse-transcription PCR; STAD, stomach adenocarcinoma.
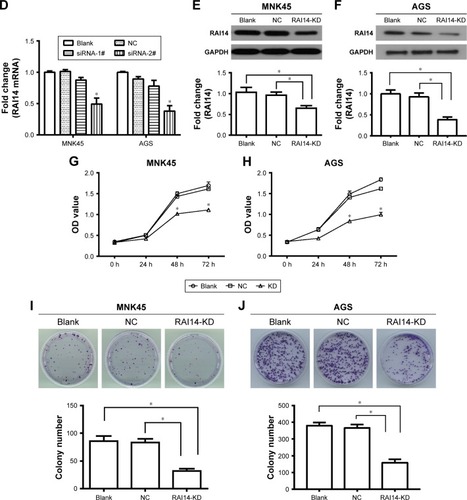
RAI14 knockdown reduces growth capacity of gastric cancer in vitro
To investigate the role of RAI14 in gastric cancer, we utilized the gastric cancer cell lines MKN45 and AGS for transfecting with RAI14 siRNA-1# and siRNA-2# or siRNA NC in order to block the expression of RAI14. The expression of RAI14 mRNA was significantly inhibited by siRAI14-2# both in MNK45 and AGS cells (P<0.05, ), and siRAI14-2# was used for subsequent experiments. Western blot was also carried out and the expression level of RAI14 protein was assessed. As evident from , RAI14 siRNA transfected cells showed a significantly decreased level of RAI14 protein as compared with the NC or blank group (P<0.05). CCK8 assay was conducted to examine the effect of RAI14 knockdown on cell proliferation in MKN45 and AGS cells. Our data showed that, following the culture for 48 hours, the proliferation ability of the siRNA-RAI14 interfered cell was clearly attenuated as compared with the NC or blank group (P<0.05, ). Moreover, the colony formation assay demonstrated that the colony formation ability was significantly decreased in RAI14 knockdown cells as compared with the NC or blank group (P<0.05, ). Taken together, as these results indicated, RAI14 knockdown significantly inhibited growth capacity of gastric cancer cells in vitro.
RAI14 knockdown inhibits cell migration and invasion of gastric cancer
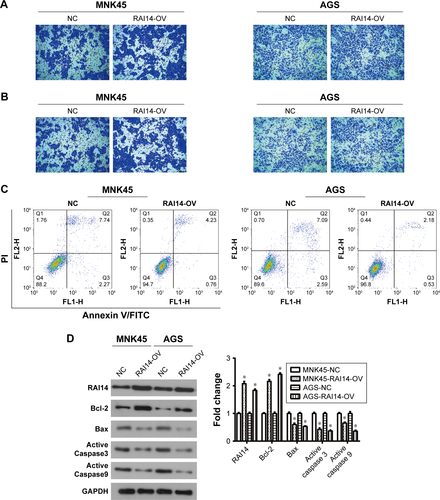
In order to investigate whether RAI14 affected tumor metastasis, a Transwell assay was adopted. As presented in , the migratory ability was both significantly decreased in RAI14 deficiency MKN45 and AGS cells as compared with the NC or blank group (P<0.01). Besides that, Transwell invasion assays revealed that RAI14 knockdown inhibited MKN45 and AGS cells invasion (). In parallel, the roles of RAI14 overexpression in MKN45 and AGS cells were also investigated. RAI14 overexpression increased the migration and invasion of gastric cancer cells in vitro (). Together, these data suggested an inhibition effect of RAI14 knockdown on migration and invasion of gastric cancer cell in vitro.
Figure 2 RAI14 knockdown inhibits cell migration and invasion, promotes apoptosis of gastric cancer cells.
Abbreviations: KD, knockdown; NC, normal control.
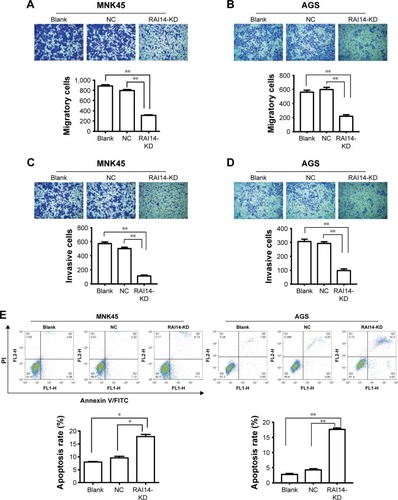
RAI14 knockdown promotes cell apoptosis of gastric cancer
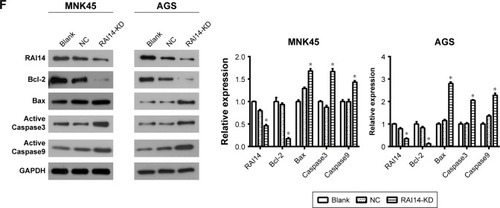
One of the significant hallmarks of tumor cells is dysregulated apoptosis, and flow cytometry was performed to examine the apoptosis rate in RAI14 knockdown cells. We observed that RAI14 knockdown significantly enhanced the apoptosis rate as compared with the NC or blank group both in MKN45 and AGS cells (P<0.05, ). Furthermore, RAI14 silencing downregulated the expression of anti-apoptotic protein Bcl-2 and upregulated the expression of pro-apoptotic protein Bax, active Caspase3 and active Caspase9 in MKN45 and AGS cells (P<0.05, ). Meanwhile, RAI14 overexpression was observed to reduce apoptosis rate in MKN45 and AGS cells (), and upregulate the expression of Bcl-2 and downregulate the expression of Bax, active Caspase3 and active Caspase9 in gastric cancer cells (P<0.05, ). Taken together, these results indicated that silencing RAI14 could accelerate apoptosis in gastric cancer cells via regulating Bcl-2/Bax axis and Caspase cascade.
RAI14 knockdown inhibits the activation of the Akt pathway in gastric cancer
In order to further explore the related mechanisms of RAI14 affecting the progression of gastric cancer, we detected changes in the Akt pathway following the RAI14 knockdown in MNK45 cells. The Akt pathway is one of the pivotal cellular pathways involved in cell proliferation, differentiation and apoptosis and participates in tumor progression and metastasis. It has been revealed that the Akt pathway is frequently activated in gastric cancer.Citation21 As the Western blot results suggest, no significant change was observed in the expression of total Akt in RAI14 knockdown MNK45 cells as compared with the NC or blank group (). Nevertheless, RAI14 knockdown significantly reduced the level of the phosphorylated form p-Akt in MKN45 cells (P<0.01, ). Meanwhile, the expression of its downstream protein Cyclin D1 was also decreased in the RAI14 deficiency MKN45 cells (P<0.01, ). It was inferred that RAI14 knockdown could inhibit the activation of Akt signaling pathway in gastric cancer. Besides that, as the Western blot results suggest, the inhibition on p-Akt by RAI14 knockdown was similar to that of LY294002, an Akt inhibitor, and it was also applied to the inhibition on proliferation (). Together with that, on the activation of Akt by IGF-1, a well-known Akt agonist, the reduction in phosphorylated of Akt and cell proliferation induced by RAI14 knockdown was significantly restored (P<0.05, ). These results highlighted that Akt pathway was involved the modulation of RAI14 in the progression of gastric cancer.
Figure 3 RAI14 knockdown inhibits the activation of Akt pathway.
Abbreviations: KD, knockdown; NC, normal control.
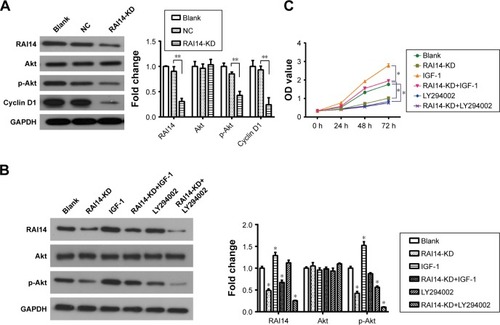
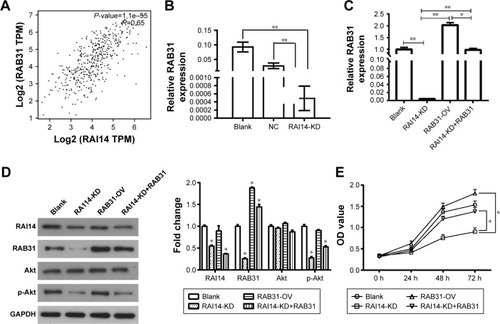
RAB31 is a potential target for RAI14
The GEPIA revealed that the expression of RAI14 was correlated with RAB31 in gastric cancer (P=1.1e-55, R=0.65, ). Our data also show that the expression of RAB31 mRNA was significantly reduced when RAI14 was knocked down in MKN45 cells (P<0.01, ). To further confirm the results, MKN45 cells were transfected with RAB31 overexpression vector (RAB31-OV) (). And RAB31 overexpression did not have significant effect on the level of RAI14 protein and p-Akt in MNK45 cells as compared with the blank group, and upregulation of RAB31 could not restore the decrease in the level of RAI14 and p-Akt caused by loss of RAI14 in MNK45 cells (). As shown in , RAB31 overexpression could significantly restore the decrease in cell proliferation induced by RAI14 knockdown (P<0.05). These results indicated that RAB31 might be a downstream target gene of RAI14 in gastric cancer.
Figure 4 RAB31 is a potential target gene for RAI14.
Abbreviations: GEPIA, gene expression profiling interactive analysis; KD, knockdown; NC, normal control; OV, overexpression.
Discussion
RAI14 has been proved as an SE associated gene that is upregulated in lung adenocarcinoma and impacts the cell proliferation.Citation11 To the best of our understanding, no data are available to indicate the relationship between RAI14 and gastric cancer. In our study, we observed that the expression of RAI14 was upregulated in gastric cancer and the RAI14 expression level was correlated with the prognosis of gastric cancer patients, indicating that RAI14 might be involved in progression of gastric cancer. This possibility has received further support of the findings that RAI14 knockdown by siRAI14 interference reduced growth capacity, migration and invasion of MKN45 and AGS cells in vitro, meanwhile, upregulation of RAI14 increased cell migration and invasion, suppressed cell apoptosis in MNK45 and AGS cells. In ovarian cancer, RAI14 is suggested to increase cell proliferation and shorten the cell cycle as a downstream gene of NR2F2.Citation23,Citation24 Yuan et alCitation11 previously reported that RAI14 is upregulated in lung adenocarcinoma cells and tissues, indicating RAI14 acts as a SE-associated oncogene in lung adenocarcinoma, while overexpression of RAI14 has an inhibitory effect on proliferation of human bronchial epithelial cell line BEAS-2B. As the above results suggest, RAI14 functions as an oncogene in the progression of gastric cancer and RAI14 deficiency could suppress growth and metastasis of gastric cancer. Nevertheless, the inconsistent role of RAI14 between tumor cells and normal cells requires further investigation, most likely owing to the diversity between tumor cells and normal cells.
Apoptosis is a pivotal regulatory mechanism for controlling cell growth, the intrinsic mitochondrial pathway is a crucial mechanism to trigger apoptosis. Escaping apoptosis is the key point for the malignant transformation and tumor progression. Herein, we worked out that RAI14 knockdown significantly promoted the apoptosis rate of MKN45 and AGS cells in vitro and downregulated the expression of Bcl-2 and upregulated the expression of Bax, active Caspase3 and Caspase9, suggesting that loss of RAI14 could induce cell apoptosis via regulating Bcl-2/Bax axis and Caspase cascade in gastric cancer.
As everyone understands, Akt pathway is involved in cell proliferation and regulation of apoptosis by impacting the expression level of downstream proteins. The Akt pathway is confirmed to be involved in tumor progression, and has been revealed to be upregulated in gastric cancer.Citation24,Citation25 Accordingly, the Akt pathway appears to be a good candidate for evaluating the mechanism of RAI14 to impair proliferation, migration and apoptosis of gastric cancer cells. We discovered that downregulation of RAI14 dramatically inhibited the activation of Akt pathway through reducing the phosphorylated form of Akt and its downstream Cyclin D1. Besides that, the reduction in proliferation caused by RAI14 knockdown was significantly restored by Akt activation stimulated by IGF-1. And the inhibition on p-Akt by RAI14 knockdown was similar to that of LY294002. Therefore, our data suggest that Akt pathway is involved in the effect of RAI14 on the progression and growth in gastric cancer. Moreover, the specific way by which RAI14 affects the Akt pathway should be explored in future research.
RAB31, a member of Rab family, is proved to be upregulated in hepatocellular carcinoma tissues, and upregulation of RAB31 could accelerate proliferation of multiple cancer cells, including glioblastoma, cervical cancer, hepatocellular carcinoma and breast cancer.Citation26–Citation28 Accordingly, knockdown of RAB31 is expected to inhibit tumor growth through decreasing cell proliferation and promoting apoptosis,Citation26,Citation27 suggesting that RAB31 plays an important role in promoting tumor progression. Herein, the regulatory pattern of RAI14 in gastric cancer was similar to RAB31, and the expression of RAI14 had a positive correlation with RAB31. We also observed that decrease in cell proliferation induced by RAI14 knockdown was rescued by RAB31 overexpression. As these results suggested, RAB31 might be a downstream target gene of RAI14 and involved in the regulation of RAI14 on the growth of gastric cancer.
Conclusion
The current study confirmed that RAI14 was aberrantly upregulated in gastric cancer and associated with prognosis. Herein, we also observed that loss of RAI14 inhibited cell proliferation, migration, invasion and survival. Moreover, RAB31 was a downstream target gene of RAI14 and involved in the regulation of RAI14 on the growth of gastric cancer. These data provide proof that RAI14 is a novel potential target for gastric cancer treatment.
Acknowledgments
This work was supported by the Natural Science Foundation of Shandong Province (ZR2014HQ071) and the Natural Science Foundation of China (81600441).
Supplementary material
Figure S1 RAI14 overexpression promotes cell migration and invasion, reduces cell apoptosis in gastric cancer cells.
Abbreviations: KD, knockdown; NC, normal control; OV, overexpression.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJSoerjomataramIDikshitRCancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012Int J Cancer20151365E359E38625220842
- StrandMSLockhartACFieldsRCGenetics of gastric cancerSurg Clin North Am201797234528325191
- ShangCSunLZhangJSilence of cancer susceptibility candidate 9 inhibits gastric cancer and reverses chemoresistanceOncotarget201789153931539828146436
- CarcasLPGastric cancer reviewJ Carcinog201413131425589897
- PengYFMandaiKSakisakaTAnkycorbin: a novel actin cytoskeleton-associated proteinGenes Cells20005121001100811168586
- KuttyRKKuttyGSamuelWMolecular characterization and developmental expression of NORPEG, a novel gene induced by retinoic acidJ Biol Chem200127642831284011042181
- KuttyRKChenSSamuelWCell density-dependent nuclear/cytoplasmic localization of NORPEG (RAI14) proteinBiochem Biophys Res Commun200634541333134116729964
- YuanWZhengYHuoRExpression of a novel alternative transcript of the novel retinal pigment epithelial cell gene NORPEG in human testesAsian J Androl20057327728816110356
- QianXMrukDDChengYHChengCYRAI14 (retinoic acid induced protein 14) is an F-actin regulatorSpermatogenesis201332
- QianXMrukDDChengCYRai14 (retinoic acid induced protein 14) is involved in regulating f-actin dynamics at the ectoplasmic specialization in the rat testisPLoS One201384e6065623565266
- YuanCHuHKuangMSuper enhancer associated RAI14 is a new potential biomarker in lung adenocarcinomaOncotarget201786210525110526129285248
- LiuHLiuXZhangSSystematic identification and annotation of human methylation marks based on bisulfite sequencing methylomes reveals distinct roles of cell type-specific hypomethylation in the regulation of cell identity genesNucleic Acids Res2016441759426635396
- WeiYZhangSShangSSEA: a super-enhancer archiveNucleic Acids Res201644D1D172D17926578594
- HniszDAbrahamBJLeeTISuper-enhancers in the control of cell identity and diseaseCell2013155493494724119843
- ShiSJWangLJYuBLiYHJinYBaiXZLncRNA-ATB promotes trastuzumab resistance and invasion-metastasis cascade in breast cancerOncotarget20156131165225871474
- BetancurPAAbrahamBJYiuYYA CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancerNat Commun201781480228378740
- ChristensenCLKwiatkowskiNAbrahamBJTargeting transcriptional addictions in small cell lung cancer with a covalent CDK7 inhibitorCancer Cell201426690992225490451
- PelishHELiauBBNitulescuIIMediator kinase inhibition further activates super-enhancer-associated genes in AMLNature2015526757227327626416749
- KwiatkowskiNZhangTRahlPBTargeting transcription regulation in cancer with a covalent CDK7 inhibitorNature2014511751161662025043025
- TangZLiCKangBGaoGLiCZhangZGEPIA: a web server for cancer and normal gene expression profiling and interactive analysesNucleic Acids Res201745W1W98W10228407145
- YingJXuQLiuBZhangGChenLPanHThe expression of the PI3K/AKT/mTOR pathway in gastric cancer and its role in gastric cancer prognosisOnco Targets Ther201582427243326366097
- HawkinsSMLoomansHAWanYWExpression and functional pathway analysis of nuclear receptor NR2F2 in ovarian cancerJ Clin Endocrinol Metab2013987E1152E116223690307
- KuttyRKChenSSamuelWCell density-dependent nuclear/cytoplasmic localization of NORPEG (RAI14) proteinBiochem Biophys Res Commun200634541333134116729964
- Al-BatranSEDucreuxMOhtsuAmTOR as a therapeutic target in patients with gastric cancerInt J Cancer2012130349149621898386
- TapiaORiquelmeILealPThe PI3K/AKT/mTOR pathway is activated in gastric cancer with potential prognostic and predictive significanceVirchows Arch20144651253324844205
- PanYZhangYChenLLiuYFengYYanJThe critical role of Rab31 in cell proliferation and apoptosis in cancer progressionMol Neurobiol20165374431443726245486
- SuiYZhengXZhaoDRab31 promoted hepatocellular carcinoma (HCC) progression via inhibition of cell apoptosis induced by PI3K/AKT/Bcl-2/BAX pathwayTumour Biol201536118661867026044564
- GrismayerBSölchSSeubertBRab31 expression levels modulate tumor-relevant characteristics of breast cancer cellsMol Cancer201211116222920728
