Abstract
Background
Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, are known to regulate cell proliferation, differentiation, apoptosis, chemotaxis, and angiogenesis. BMPs also participate in the development of most tissues and organs in vertebrates. Recombinant human (rh) BMPs, such as rhBMP-2, rhBMP-4, and rhBMP-7, have been recently approved to augment spinal fusion and recalcitrant long-bone non-unions because of their equivalent or superior efficacy to autogenous bone graft in enhancing bony fusion. Nonetheless, the use of BMPs is contraindicated in surgery for bone tumors because of concerns that this anabolic growth factor may cause tumor proliferation. However, we have repeatedly reported that BMP-2 is effective in inducing osteogenic differentiation of a subpopulation of osteosarcoma (OSA) cells that acquire stem cell attributes and are capable of reconstituting tumor masses, which in turn suppress the malignancy of the bone tumor.
Methods
3×105/20 µL human OSA 143B cells were inoculated into 5–6 weeks old BABL/c nude mice to establish orthotopic OSA. X-ray device was used to monitor the developed tumors in animals. Necropsy was performed and the pathology of lung metastasis were tested by Haemotoxylin and Eosin. Moreover, bone formation induced by rhBMP-2 was investigated through micro-computed tomography. In addition, immunohistochemistry staining was used to evaluate the tumorigenicity and growth of OSA cells after rhBMP-2 treatment.
Results
In the present study, we established an orthotopic model of OSA by inoculating 143B cells into BABL/c mice, which resulted in a tumor occurrence rate of 100%. Following the treatment with rhBMP-2, lung metastasis, which contributes to poor prognosis, was significantly restricted, indicating an additional aspect of rhBMP-2 to suppress expansion of OSA. Concurrently, our micro-computed tomography and radiographic analyses showed that rhBMP-2 reduced the invasion of tumor cells into adjacent bone tissue, which in turn helped to preserve the integrity of the affected bone tissue. Finally, the growth of Ki-67-positive cells and those cells that express high levels of aldehyde dehydrogenase (ALDHbr) was found to be inhibited in the developed tumors.
Conclusion
On the basis of these results, we conclude that rhBMP-2 can impede the malignancy of OSA by reducing lung metastasis of the tumor. Induction of the tumor cells by rhBMP-2 also helps to preserve the impaired skeleton. These results imply that BMP-2 or BMP-2-mimetic drugs, if properly combined with traditional therapies, may provide a new therapeutic option for the treatment of OSA.
Introduction
Osteosarcoma (OSA) is one of the most malignant tumors particularly occurring in children, adolescents, and young adults.Citation1–Citation6 Over 60% of patients with OSA are diagnosed between the age of 10–20 years old,Citation7 with tumor present in the spine and limbs such as distal femur, proximal tibia, and proximal humerus.Citation5 OSA in elderly patients, on the other hand, is often linked to other causes such as Paget’s disease or some other bone lesions.Citation5,Citation7,Citation8 Over the past few decades, the combined use of chemotherapeutics with aggressive surgery has improved the long-term survival rates of patients with OSA to around 65%.Citation9,Citation10 However, approximately 10%–20% of patients with OSA have metastases at the initial diagnosis. When the tumor metastasizes to the lung, almost all patients succumb to death, unless the pulmonary metastasis can be resected completely.Citation11 Clinically, metastatic OSA also poses a great challenge for its treatment. Signatures of genes that regulate chemoresistance are often expressed in the metastatic tumor.Citation12,Citation13 Furthermore, conventional chemotherapy is always accompanied with toxic and adverse effects that compromise the quality of life of patients.Citation14–Citation17
Reconstruction of impaired skeleton affected by OSA presents an additional challenge. Surgical management of symptomatic OSA typically involves resection of the tumor in conjunction with bone stabilization. Such stabilization presently involves placement of instrumentation to internally fixate the affected segments. Instrumentation, however, will eventually fail unless a bony fusion occurs. Unfortunately, the patients with OSA typically also undergo chemo- and radiation therapies that negatively affect such fusion. Even without adjuvant treatments, bone regeneration can still be difficult to achieve with standard fusion techniques. All these hurdles have urged new investigations to explore new alternatives to treat OSA.
Bone morphogenetic proteins (BMPs), a group of cytokines belonging to the TGF-β superfamily, play important roles in bone tissue formation and remodeling.Citation18–Citation20 BMPs have been known to regulate cell proliferation, apoptosis, differentiation, chemotaxis, and angiogenesis and induce bone and cartilage formation in vivo and in vitro.Citation18,Citation21–Citation24 However, the roles of BMPs in tumorous tissues are often contradictory. BMP-2 has been shown to stimulate the growth of pancreatic carcinoma and lung carcinoma.Citation25,Citation26 On the other hand, BMP-2 clearly inhibits the growth of tumor cells including breast cancer,Citation27,Citation28 myeloma,Citation29 gastric cancer,Citation30 colon cancer,Citation31 brain tumor,Citation32 and prostate cancer in the absence of androgen.Citation33,Citation34 Overexpression of BMP-2 in rat OSA UMR106 cells inhibits proliferation and results in osteoblastic transdifferentiation.Citation35 Geng et al have shown that Coleusin factor inhibits OSA proliferation through BMP-2 upregulation and subsequent osteoblastic differentiation.Citation36 Wang et al reported that BMP-2 causes inhibition of tumor-inducing gene expression and upregulation of differentiation markers, which in turn inhibit the tumorigenicity of high aldehyde dehydrogenase expressed (ALDHbr) cells derived from human OSA OS99-1 cells.Citation37 Recombinant human (rh) BMP-2 has also been reported to inhibit tumor growth and induce bone formation in human renal cell carcinoma cells,Citation38 which often invade spinal columns due to the close vicinity. The exogenous application of BMP-2 to the surgical site following tumor excision does not increase the risk of local recurrence.Citation39 Furthermore, a recent study by Gill et al using xenografted OSA indicated that the addition of BMP-2 did not increase the risk of lung metastasis.Citation40 However, the major limitation of all the above studies was that the observations were mainly based on the subcutaneous model system. It will therefore be more clinically beneficial and relevant to conduct the evaluation with an orthotopic model to further improve the efficacy of BMP-2 in the treatment of OSA.
In the present study, an orthotopic OSA model with a high lung metastasis rate was established. The affected animals expressed several clinical symptoms similar to those observed in human patients.Citation41 We then used the model to further assess the effect of BMP-2 on lung metastasis and how it can be used to treat bone erosion symptoms.
Materials and methods
Cell culture and inoculation of OSA cells
Human OSA 143B cell lines were purchased from the American Type Culture Collection. Cells were cultured in minimum essential medium (Hyclone) supplemented with 10% FBS (Gibco) in a humidified atmosphere of 5% CO2 in air at 37°C and used during the log phase of growth. A total of 3×105/20 µL human OSA 143B cells were inoculated into 5- to 6-week-old BABL/c nude mice (SLRC Laboratory Animal) to establish orthotopic OSA. The animals were anesthetized with Zoletil 50 (Virbac), and operative fields were prepared with iodine and 75% alcohol. OSA cells were directly injected into the right side of the proximal tibia. All the studies were approved and performed according to the protocols of Institutional Animal Care and Use Committee of Beihang University.
OSA models treated with rhBMP-2
BABL/c nude mice with orthotopic OSA were divided into four groups at random: Group A (n=14, 17.76±0.48 g); Group B (n=14, 17.81±0.65 g); Group C (n=14, 17.69±0.55 g); and Group D (n=15, 17.97±0.56 g). For the rhBMP-2/collagen gel preparation, 25 µL of injectable collagen gel was mixed with 2.5 µL of the same reconstituted solution containing 2.5 µg of rhBMP-2 (#355-BM; R&D Systems). The compound was then injected into the animals of Groups C and D 14 days after tumor cell inoculation of 143B cells with a 0.3 mm (25-gauge) needle at the orthotopic sites. The animals were anesthetized as described before. All the tumor-bearing mice were sacrificed after 42 or 56 days of tumor cell inoculation. The animals identified to have critical conditions before this period were excluded early from the study and humanely sacrificed.
Radiographic track of the developed OSA
A portable X-ray device (BJI-G; HSCreate) was used to monitor the developed tumors in animals. The entire affected limbs were monitored every 7 days with animals under anesthesia as aforementioned.
Lung metastasis assay
After the animals were sacrificed, their lung tissues were collected and stained with hematoxylin and eosin. Necropsy was performed, and the pathology of lung metastasis was graded according to the number and size of the identified tumor nodules in the lung tissues. Samples were categorized according to the following scores: 0= relatively normal where no tumor cell aggregation is present in the pulmonary tissue; 1= mild where tumor cell aggregation is <25% coverage; 2= moderate indicating the aggregation region accounts for 25%–50%; 3= moderate to marked where the aggregation coverage extends to 50%–75%; 4= marked where >75% aggregation is distributed in the affected lung tissue.
Micro-computed tomography to scan bone tissue
Micro-computed tomography was performed postmortem by using the SkyScan1176 system (Bruker microCT) at 42 days (Groups A and C) and 56 days (Groups B and D) after cell inoculation. Scans were performed at the tibiae using a 0.5 mm aluminum filter at 50 kV voltage and 500 µA current. Images were then reconstructed using Mimics®17.0 (Materialise). Bone mineral content (BMC), volumetric bone mineral density (vBMD), bone volume (BV), and bone surface (BS) were calculated, and the region of interest was 400 slices from the growth plate to the distal tibia.
Immunohistochemical staining
Cells expressing a high level of ALDHbr and Ki-67 were stained. Briefly, formalin-fixed paraffin-embedded sections of tumor samples were stained with mouse anti-Ki-67 antibody (sc-23900; Santa Cruz Biotechnology, Inc.) and mouse anti-ALDH antibody (sc-166362; Santa Cruz Biotechnology, Inc.) using standard procedures. Immunofluorescent staining was performed as previously described.Citation42
Statistical analysis
Data were expressed as mean ± SD. Statistically significant differences were determined by two-tailed Student’s t-test using SPSS 22 software (IBM, Armonk, NY, USA) with a significant level at P<0.05.
Results
An orthotopic OSA model with high tumor incidence
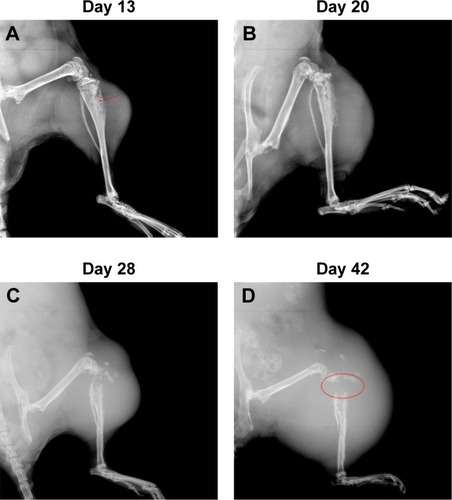
Fourteen days after implantation with 143B human OSA cells into the tibia, eight mice were randomly photographed. The results confirmed that the tumor growth rate had reached almost 100% (). Radiographic evidence indicated osteolytic epiphyses, with the presence of the Codman triangle, which is a primary characteristic of OSA observed in bone tissue (). Further, erosion of the affected skeleton deteriorated over time (). Skeleton of the affected limbs was severely eroded, and the affected soft tissue formed few lumps with calcification shadows 28 days later (). With progression in time, the proximal tibia tissue was completely impaired, and the adjacent soft lumps continued to grow until 42 days (). In addition, the first spontaneous death occurred in Group B (untreated) after 37 days of inoculation of tumor cells.
Figure 1 The developed mouse model of osteosarcoma (orthotopic).
Figure 2 Radiographs indicated the impaired proximal tibias by OSA.
Abbreviation: OSA, osteosarcoma.
Restriction of lung metastasis by rhBMP-2
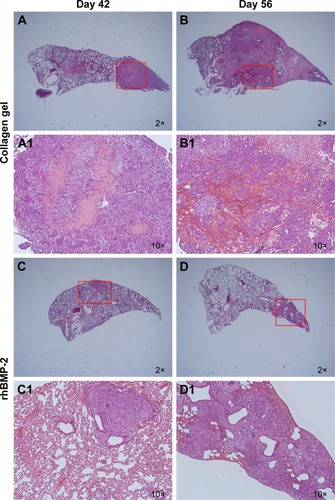
Metastatic nodules were observed in the sections of lung tissues and presented in different numbers and sizes. Large tumor nodules were identified to colonize the lung tissue of BABL/c mice without rhBMP-2 treatment at 42 days (). Eventually, the entire organ was infiltrated by the tumor cells as shown in . Although lung metastasis also occurred in the groups treated with rhBMP-2, the tumor nodules were much smaller and confined without extensive spread ().
Figure 3 Histopathological appearance of lung metastasis nodules.
Abbreviations: rhBMP-2, recombinant human BMP-2; OSA, osteosarcoma.
To evaluate whether rhBMP-2 restricts the development of metastatic tumors in the lung, the number and size of nodules identified in the tissue sections were measured (coverage of tumor cell aggregation) to assess the efficacy. Metastasis grading () indicated no obvious difference between the treated and untreated groups (Groups A and C) at 42 days, with grades of 2.4±1.14 (n=5) and 2.0±1.0 (n=5), respectively. However, metastasis was highly inhibited at 56 days in the group receiving rhBMP-2 (Group D), with a grade of 2.2±1.48 (n=5), compared to the untreated group (Group B) that developed marked lung metastasis, with a grade of 3.8±0.45 (n=5).
Table 1 GradingTable Footnotea of lung metastasis of OSA-bearing animals
Bone formation induced by rhBMP-2 in tumor-bearing mice
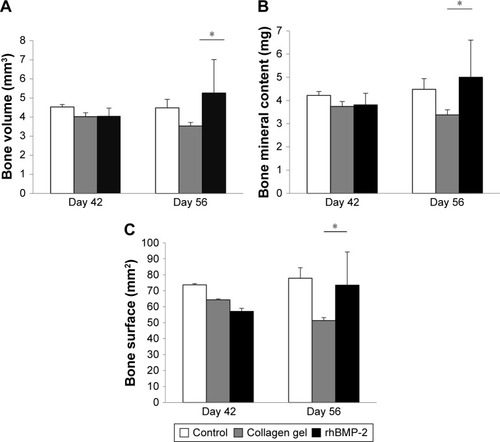
As shown in , three-dimensional (3D) reconstruction of tibia micro-architecture shows that the proximal tibia was completely eroded in the untreated groups (Groups A and B) at 42 days after inoculation with tumor cells. The impaired skeleton had deteriorated and developed structural failure at 56 days (). On the other hand, the integrity of the affected skeleton was much preserved with structured bone formation in Groups C and D that were treated with rhBMP-2 ().
Figure 4 rhBMP-2 reduced osteolysis and protected the integrity of the affected bone tissue.
Abbreviation: rhBMP-2, recombinant human BMP-2.
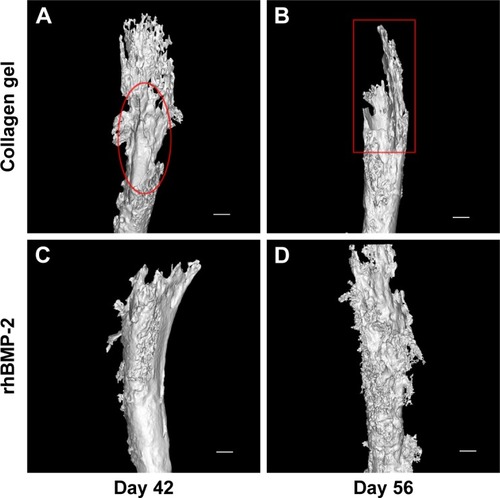
In addition, as shown in , the defined image parameters used to assess bone quality were significantly higher in the treated group, including BV (Group B =3.35±0.19 mm3, Group D =5.26±1.75, P<0.05), BMC (Group B =3.38±0.22 mg, Group D =5.0±1.6, P<0.05), and BS (Group B =51.27±1.93 mm2, Group D =73.5±20.78, P<0.05). Note that the assessed values of BV, BMC, and BS in Group D were even comparable to those in the normal control ().
Table 2 Microstructural properties of the approached tibias analyzed by micro-computed tomography
Tumorigenicity and growth of OSA cells inhibited by rhBMP-2
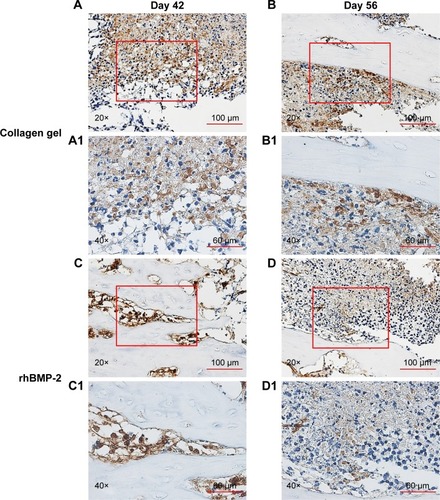
Immunohistochemical staining of the tumor sections confirmed that the growth of cells expressing a high level of aldehyde dehydrogenase (ALDHbr), a putative marker for a variety of cancer stem cells (CSCs),Citation42,Citation43 was suppressed in groups treated with rhBMP-2 compared to that in the untreated ones (). Ki-67-positive cells (highly proliferating cells) were also greatly reduced by BMP-2 treatment, whereas the expression of Ki-67 was much higher in Group C (untreated) at 42 days ().
Figure 6 Immunohistochemical staining of ALDHbr cells.
Abbreviations: rhBMP-2, recombinant human BMP-2; ALDHbr, high aldehyde dehydrogenase expressed.
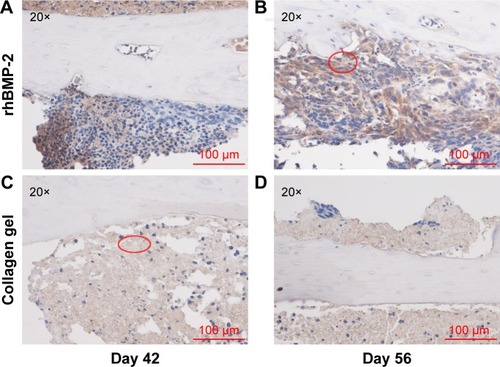
Figure 7 Reduction of Ki-67 positive cells by BMP-2 treatment.
Abbreviation: rhBMP-2, recombinant human BMP-2.
Discussion
OSA is the most common primary bone malignancy of childhood and adolescence, comprising almost 60% of the common histological subtypes of bone sarcomas in childhood.Citation44 Despite the success of neoadjuvant chemotherapy for OSA to improve prognosis, OSA still has one of the lowest survival rates for pediatric cancer because of local recurrence and metastasis. Continuous efforts using immunotherapies,Citation45,Citation46 tissue factors,Citation47 and/or surgical resectionsCitation48 have been suggested to reduce lung metastasis; yet, the mechanisms of these strategies have not been fully elucidated. Increasing evidence supports the hypothesis that tumors are organized in a hierarchy of heterogeneous cell populations with different proliferative potentials. The capability to initiate tumor formation and promote tumor growth exclusively resides in a small subpopulation of tumor cells, termed CSCs or tumor-initiating cells. Many studies have confirmed that CSCs are responsible for tumor progression, metastasis, recurrence, and resistance to chemotherapy and radiation treatments.Citation49–Citation52 Experimental evidence also supports the notion that OSA are organized and sustained by CSCs.Citation42,Citation43,Citation53,Citation54
According to a maturation arrest model proposed by Sell,Citation55,Citation56 CSCs do not undergo typical turnover like mature cells and remain present before proliferative stimulus is given. This model also explains that the differentiation state of the cancer that develops will depend on the stage of differentiation at which maturation arrest occurs, thereby clarifying the cause of poor prognosis with lowly differentiated tumor cells.Citation56 Differentiation therapy has been proposed to induce terminal differentiation of CSCs, thereby depriving CSCs of the self-renewal property and chemoresistance. Extensive studies have shown that CSCs are chemoresistant until they are differentiated into amplifying “progenies”, which become sensitive to chemotherapy.Citation57 In this aspect, differentiation therapy holds great promise for cancer treatment and has been supported by many studies.Citation58–Citation61 Yan et al confirmed that overexpression of IKKa induces differentiation and reduces tumorigenicity of nasopharyngeal carcinoma cells.Citation62 Hepatocellular carcinoma (HCC)-derived CSCs could be differentiated into hepatocytes and neuronal cells by induction with the sex-determining factor.Citation63 Other researchers also demonstrated that BMP-4 promotes differentiation of HCC-derived CSCs and in turn inhibits the self-renewal, chemotherapeutic resistance, and tumorigenic capacity of these cells.Citation64 BMP-4 has also been reported to inhibit tumorigenic potential of human brain tumor-initiating cells.Citation32 Campos et al revealed that all-trans retinoic acid (ATRA) induced differentiation of stem cell-like glioma cells, leading to reduced motility and tumorigenicity of CSCs in vitro.Citation65 ATRA treatment was also found to be effective to induce differentiation of breast cancer cells, resulting in reduced invasiveness and migration, and increased sensitivity to anticancer treatment.Citation66 Wang et al suggested that CSCs in OSA can be induced by BMP-2 toward osteogenic differentiation that in turn drastically restricts their tumorigenecity,Citation37,Citation42,Citation43,Citation67 thereby supporting the early concept to introduce BMP-2 as a differentiation therapy for treating OSA.
In the present study, we aimed to use an orthotopic model to further our investigation of the proposed differentiation therapy with BMP-2 that restricts lung metastasis. A high tumor formation rate and pulmonary metastasis were noted to be associated with the developed model. This was very beneficial for assessing the efficacies of new therapeutics to treat OSA as the model replicates the critical symptoms of the tumor as well as exhibits a high incidence of tumor occurrence. However, as reported earlier, there was spontaneous death occurring within 40 days. The short survival time of tumor-bearing animals can be attributed to high concentration of the injected tumor cells as indicated elsewhere.Citation41
As shown histopathologically, the metastatic tumor in lung tissues was suppressed in the rhBMP-2-treated animals for a longer period of time compared to that in the untreated groups. In a similar study reported by Gill et al, the authors concluded that when treated with BMP-2, either single dose or multiple doses, there was no difference in the incidence of lung metastasis in animals developing OSA; this finding was consistent with our observation in the present study.Citation40
In addition, our results also showed that BV and BMC, the most indicational parameters of bone quantity, were significantly increased in the tumor-bearing animals treated with rhBMP-2 at 56 days. This was consistent with the rendered, reconstructed 3D images of the approached tibiae, where non-diffuse new bone formation was observed in the treatment groups. In contrast, large and extensive bone defects were found in the untreated groups. These results suggest that the treatment with rhBMP-2 induced bone formation in the OSA-impaired skeleton, which helps to preserve the integrity of the affected bone tissue in the tumor-bearing mice, thus suggesting a bone protective effect of the investigated regimen for the tumor-affected bone tissue.
Our data also showed that ALDHbr cellsCitation42,Citation43 and Ki-67-positive cells were decreased with the treatment, indicating that the tumor expansion was restricted by rhBMP-2. However, it was noted that Ki-67-positive cells were still highly present at the early stage during the treatment. This phenomenon corresponds to the Sell’s model of maturation arrest, in which the more differentiated progenies of CSCs undergo an amplifying stage to expand the tumor and maintain its heterogenicity.Citation55,Citation56 With the delivered rhBMP-2 in the vicinity, these cells became more differentiated and fate-determined with downregulated ALDH expression and proliferative capacity. These results were consistent with the findings reported by Wang et al wherein CSCs and their low aldehyde dehydrogenase-expressing progenies were induced to form calcified and structured bone tissue with much restricted proliferating cells.Citation37,Citation42,Citation43,Citation67,Citation68
Although the current study additionally presents an encouraging promise to incorporate BMP-2 and/or its analogies into the current regimens to treat OSA, further investigations are necessary to elucidate the proposed mechanism. First, as the early data presented in this study show a tendency for rhBMP-2 to suppress lung metastasis of OSA, it will be more clinically meaningful to see how this correlates with the survival rate of the recruited animals. Second, previous studies have shown that the expression of key CSC markers such as Oct3/4a, Nanog, and Sox-2 genes is downregulated with elevated expression of osteogenic markers such as Runx-2, ALP, and collagen type I both in vitro and in vivo (subcutaneously) when the OSA cells are exposed to rhBMP-2.Citation37,Citation43,Citation67 Given the complexity of the bone microenvironment in situ, it certainly warrants more detailed studies by recovering cells from the affected skeleton of the animals for molecular probing. We believe that this will help to understand how the cells might follow the proposed pathways to respond to BMP-2 treatment as reported previously.Citation37,Citation42,Citation67 Last, it is also of our interest to perform biomechanical testing of the affected limb to assess the level of functional preservation of the affected bone tissue, such as its mechanical strength and material properties.
Acknowledgments
The authors acknowledge all the participants and participating institutes for experimental and technical support in this study. The present study was supported by the National Natural Science Foundation of China (NSFC grant no 31470045).
Disclosure
The authors report no conflicts of interest in this work.
References
- PicciPOsteosarcoma: What did we learn from the paediatric experience for adolescents and young adults?European Journal of Cancer Supplements200755227234
- SweetnamROsteosarcomaBr J Hosp Med19822821121161216957252
- DorfmanHDCzerniakBBone cancersCancer1995751 Suppl2032108000997
- OttavianiGJaffeNThe epidemiology of osteosarcomaCancer Treat Res200915231320213383
- MirabelloLTroisiRJSavageSAOsteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results ProgramCancer200911571531154319197972
- WangLLBiology of osteogenic sarcomaCancer J200511429430516197719
- EkETOjaimiJKitagawaYChoongPFOutcome of patients with osteosarcoma over 40 years of age: is angiogenesis a marker of survival?Int Semin Surg Oncol20063716551370
- HuvosAGOsteogenic sarcoma of bones and soft tissues in older persons. A clinicopathologic analysis of 117 patients older than 60 yearsCancer1986577144214492418941
- MeyersPASchwartzCLKrailoMDOsteosarcoma: the addition of muramyl tripeptide to chemotherapy improves overall survival – a report from the Children’s Oncology GroupJ Clin Oncol200826463363818235123
- DamronTAWardWGStewartAOsteosarcoma, chondrosarcoma, and Ewing’s sarcoma: National Cancer Data Base ReportClin Orthop Relat Res2007459404717414166
- KagerLZoubekAPötschgerUPrimary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocolsJ Clin Oncol200321102011201812743156
- MintzMBSowersRBrownKMAn expression signature classifies chemotherapy-resistant pediatric osteosarcomaCancer Res2005655651754
- Endo-MunozLCummingASommervilleSDickinsonISaundersNAOsteosarcoma is characterised by reduced expression of markers of osteoclastogenesis and antigen presentation compared with normal boneBr J Cancer20101031738120551950
- HaydonRCZhouLFengTNuclear receptor agonists as potential differentiation therapy agents for human osteosarcomaClin Cancer Res2002851288129412006550
- ChouAJGorlickRChemotherapy resistance in osteosarcoma: current challenges and future directionsExpert Rev Anticancer Ther2006671075108516831079
- ClarkJCDassCRChoongPFA review of clinical and molecular prognostic factors in osteosarcomaJ Cancer Res Clin Oncol2008134328129717965883
- JanewayKAGrierHESequelae of osteosarcoma medical therapy: a review of rare acute toxicities and late effectsLancet Oncol201017670678
- WozneyJMRosenVCelesteAJNovel regulators of bone formation: molecular clones and activitiesScience19882424885152815343201241
- ChenGDengCLiYPYpLTGF-β and BMP signaling in osteoblast differentiation and bone formationInt J Biol Sci20128227228822298955
- YeLMasonMDJiangWGBone morphogenetic protein and bone metastasis, implication and therapeutic potentialFront Biosci201116865
- HruskaKAMathewSSaabGBone morphogenetic proteins in vascular calcificationCirc Res200597210511416037577
- HoganBLBone morphogenetic proteins: multifunctional regulators of vertebrate developmentGenes Dev19961013158015948682290
- BarbozaECaúlaAMachadoFPotential of recombinant human bone morphogenetic protein-2 in bone regenerationImplant Dent19998436036710709481
- ThawaniJPWangACThanKDLinCYLa MarcaFParkPBone morphogenetic proteins and cancer: review of the literatureNeurosurgery201066223324620042986
- KleeffJMaruyamaHIshiwataTBone morphogenetic protein 2 exerts diverse effects on cell growth in vitro and is expressed in human pancreatic cancer in vivoGastroenterology199911651202121610220513
- LangenfeldEMCalvanoSEAbou-NuktaFLowrySFAmentaPLangenfeldJThe mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cellsCarcinogenesis20032491445145412819188
- Ghosh-ChoudhuryNWoodruffKQiWCelesteAAbboudSLGhosh ChoudhuryGBone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylationBiochem Biophys Res Commun2000272370571110860819
- Ghosh-ChoudhuryNGhosh-ChoudhuryGCelesteABone morphogenetic protein-2 induces cyclin kinase inhibitor p21 and hypophosphorylation of retinoblastoma protein in estradiol-treated MCF-7 human breast cancer cellsBiochim Biophys Acta20001497218619610903423
- KawamuraCKizakiMYamatoKBone morphogenetic protein-2 induces apoptosis in human myeloma cells with modulation of STAT3Blood20009662005201110979940
- WenXZMiyakeSAkiyamaYYuasaYBMP-2 modulates the proliferation and differentiation of normal and cancerous gastric cellsBiochem Biophys Res Commun2004316110010615003517
- BeckSEJungBHFiorinoABone morphogenetic protein signaling and growth suppression in colon cancerAm J Physiol Gastrointest Liver Physiol20062911G135G14516769811
- PiccirilloSGReynoldsBAZanettiNBone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cellsNature2006444712076176517151667
- IdeHYoshidaTMatsumotoNGrowth regulation of human prostate cancer cells by bone morphogenetic protein-2Cancer Res19975722502250279371496
- BrubakerKDCoreyEBrownLGVessellaRLBone morphogenetic protein signaling in prostate cancer cell linesJ Cell Biochem200491115116014689587
- HuangWRudkinGHCarlsenBOverexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cellsExp Cell Res2002274222623411900483
- GengSSunBLuRWangJColeusin factor, a novel anticancer diterpenoid, inhibits osteosarcoma growth by inducing bone morphogenetic protein-2-dependent differentiationMol Cancer Ther20141361431144124723453
- WangLParkPZhangHBMP-2 inhibits the tumorigenicity of cancer stem cells in human osteosarcoma OS99-1 cell lineCancer Biol Ther201111545746321178508
- WangLParkPZhangHBMP-2 inhibits tumor growth of human renal cell carcinoma and induces bone formationInt J Cancer201213181941195022275155
- GellerDSSinghMYZhangWDevelopment of a model system to evaluate local recurrence in osteosarcoma and assessment of the effects of bone morphogenetic protein-2Clin Cancer Res201521133003301225472999
- GillJConnollyPRothMThe effect of bone morphogenetic protein-2 on osteosarcoma metastasisPLoS One2017123e017332228264040
- YuZSunHFanQLongHYangTMaBEstablishment of reproducible osteosarcoma rat model using orthotopic implantation techniqueOncol Rep20092151175118019360291
- WangLParkPZhangHLa MarcaFLinCYProspective identification of tumorigenic osteosarcoma cancer stem cells in OS99-1 cells based on high aldehyde dehydrogenase activityInt J Cancer2011128229430320309879
- WangLParkPLinCYCharacterization of stem cell attributes in human osteosarcoma cell linesCancer Biol Ther20098654355219242128
- GattaGCapocacciaRStillerCChildhood cancer survival trends in Europe: a EUROCARE Working Group studyJ Clin Oncol200523163742375115923571
- ShimizuTFuchimotoYFukudaKOkitaHKitagawaYKurodaTThe effect of immune checkpoint inhibitors on lung metastases of osteosarcomaJ Pediatr Surg201752122047205028954696
- SarafAJFengerJMRobertsRDOsteosarcoma: Accelerating Progress Makes for a Hopeful FutureFront Oncol20188429435436
- TiekenCVerboomMCRufWTissue factor associates with survival and regulates tumour progression in osteosarcomaThromb Haemost201611551025103326763081
- HeatonTEDavidoffAMSurgical treatment of pulmonary metastases in pediatric solid tumorsSemin Pediatr Surg201625531131727955735
- PardalRClarkeMFMorrisonSJApplying the principles of stem-cell biology to cancerNat Rev Cancer200331289590214737120
- JordanCTGuzmanMLNobleMCancer stem cellsN Engl J Med2006355121253126116990388
- ClarkeMFDickJEDirksPBCancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cellsCancer Res200666199339934416990346
- DeanMFojoTBatesSTumour stem cells and drug resistanceNat Rev Cancer20055427528415803154
- Basu-RoyUBasilicoCMansukhaniAPerspectives on cancer stem cells in osteosarcomaCancer Lett2013338115816722659734
- AbarrategiATorninJMartinez-CruzadoLOsteosarcoma: Cells-of-Origin, Cancer Stem Cells, and Targeted TherapiesStem Cells Int201620161313
- SellSStem cell origin of cancer and differentiation therapyCrit Rev Oncol Hematol200451112815207251
- SellSCancer stem cells and differentiation therapyTumour Biol2006272597016557043
- AbdullahLNChowEKMechanisms of chemoresistance in cancer stem cellsClin Transl Med2013213323369605
- MassardCDeutschESoriaJCTumour stem cell-targeted treatment: elimination or differentiationAnn Oncol200617111620162416600978
- AguadoTCarracedoAJulienBCannabinoids induce glioma stem-like cell differentiation and inhibit gliomagenesisJ Biol Chem200728296854686217202146
- YanMLiuQDifferentiation therapy: a promising strategy for cancer treatmentChin J Cancer201635326739838
- JinXJinXKimHCancer stem cells and differentiation therapyTumour Biol20173910101042831772993329072131
- YanMZhangYHeBIKKα restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiationNat Commun20145366124739462
- MurakamiSNinomiyaWSakamotoEShibataTAkiyamaHTashiroFSRY and OCT4 Are Required for the Acquisition of Cancer Stem Cell-Like Properties and Are Potential Differentiation Therapy TargetsStem Cells20153392652266326013162
- ZhangLSunHZhaoFBMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinomaCancer Res201272164276428522773665
- CamposBWanFFarhadiMDifferentiation therapy exerts antitumor effects on stem-like glioma cellsClin Cancer Res201016102715272820442299
- YanYLiZXuXAll-trans retinoic acids induce differentiation and sensitize a radioresistant breast cancer cells to chemotherapyBMC Complement Altern Med20161611327036550
- WangLParkPLa MarcaFThanKRahmanSLinCYBone formation induced by BMP-2 in human osteosarcoma cellsInt J Oncol20134341095110223900689
- WangLParkPLa MarcaFThanKDLinCYBMP-2 inhibits tumor-initiating ability in human renal cancer stem cells and induces bone formationJ Cancer Res Clin Oncol201514161013102425431339