Abstract
Purpose
CLIC1, a member of the highly conserved class ion-channel protein family, is frequently upregulated in multiple human malignancies and has been demonstrated to play a critical role in cell proliferation, apoptosis, and invasion. However, limited is known about its expression, biological functions, and action mechanism in oral malignancies. We aimed to evaluate whether CLIC1 could be a biomarker for oral squamous cell carcinoma (OSCC).
Methods
Immunohistochemistry was used to analyze the expression of CLIC1 in tissue. CLIC1 protein and mRNA were measured through Western immunoblotting and quantitative real-time PCR. CLIC1 protein expression in plasma was detected via ELISA. A total of 72 OSCC specimens were recruited in this study for evaluation of correlations of CLIC1 with clinicopathological features and survival.
Results
CLIC1 was significantly overexpressed in tissue and plasma of OSCC patients. It was found that upregulated CLIC1 was distinctly correlated with histological grade, TNM stage, and tumor size. Meanwhile, Kaplan–Meier survival analysis showed that OSCC patients with high CLIC1 expression had remarkably poorer overall survival rate than those with low CLIC1 expression. Multivariate Cox regression analysis revealed that CLIC1 was the independent prognostic factor for overall survival rate of OSCC patients. In addition, Pearson correlation analysis showed that CLIC1 was associated with multiple tumor-associated genes.
Conclusion
These results indicated that CLIC1 acts as a molecular target in OSCC and may present a novel diagnostic marker and therapeutic target for OSCC.
Introduction
It has been reported that oral squamous cell carcinoma (OSCC) is the commonest oral malignancy, with ~300,000 new cases worldwide annually.Citation1 Although tremendous progress has been achieved in radical surgery combined with postoperative radiotherapy/chemoradiotherapy, patient 5-year survival rate has not improved essentially in recent decades. Therefore, early detection, early diagnosis, and early treatment are very important to increase the 5-year survival rate of patients with OSCC. Novel and specific biomarkers are required to solve this problem.
Molecular changes are regarded as potential biomarkers for cancers. In OSCC, some genes have been reported as potential biomarkers, such as KPNA2, HOXA13, HOXD13, EZH2, and LEF1.Citation2–Citation5 They had certain value in the diagnosis, treatment, and prognosis of OSCC, but it was not enough to obtain sensitivity and specificity for OSCC only by them. CLIC1 is a member of the highly conserved category of chloride-channel proteins that exists in both integral membrane and soluble forms.Citation6 It is abundant in the apical domains of epithelia.Citation7 Recently, CLIC1 has been reported to be responsible for regulating the migration, invasion, apoptosis, and drug sensitivity of cancer cells and is involved in several signaling pathways, such as MAPK–ERK, PI3K–Akt, and MAPK–p38 ().Citation8–Citation24 However, whether CLIC1 plays an important role in tumorigenesis and progression of OSCC remains elusive.
Table 1 Function of CLIC1 in different cancers
To determine the role of CLIC1 in OSCC, we examined CLIC1 mRNA and protein expression in OSCC tissues and plasma. Moreover, we analyzed the association between CLIC1 expression and clinicopathological characteristic in OSCC. In addition, we elucidated the prognostic role of CLIC1 in OSCC patients. Finally, we found that CLIC1 was associated with multiple tumor-associated genes.
Methods
Sample preparation and clinicopathological data
An immunohistochemistry analysis was conducted on OSCC specimens collected from 72 patients who had undergone radical surgical resection (without prior radiotherapy or chemotherapy) from January 2012 to February 2013 at the Research Center of Pathology, Chongqing Medical University. A total of 26 pairs freshly frozen OSCC and corresponding noncancerous tissue samples were used for quantitative real-time (qRT) PCR and Western blot; 76 plasma samples included 54 OSCC plasma samples collected from OSCC patients and 22 healthy controls. All tissue and blood samples were collected at the Stomatological Hospital of Chongqing Medical University, China from 2017 to 2018 and used for qRT-PCR, Western blotting, and ELISA analysis. This study was approved by the Ethics Committee of the Stomatological Hospital of Chongqing Medical University, and all patients provided informed written consent before enrollment. Collection and processing of tissue and blood samples were conducted in accordance with the Declaration of Helsinki.
All tissue samples and blood samples were collected from patients and stored using the standard procedure.Citation25 These samples had undergone only one freeze–thaw cycle before the study was carried out. Telephone interviews were conducted routinely for at least 6 months during follow-up. Time from diagnosis to death or last follow-up visit was regarded as survival time and was checked at the last telephone interview in February of 2018, regardless of whether the patient was alive or not.
Immunohistochemistry
Standard immunoperoxidase staining was used for immunohistochemical staining and CLIC1 protein expression in normal and malignant samples evaluated. Each section was semiquantitatively scored based on the extent and intensity of immunoreactivity: 0, 10% immunoreactive cells; 1, ≤25% immunoreactive cells; 2, 25%–75% immunoreactive cells; and 3, >75% immunoreactive cells. Additionally, staining intensity was graded on a 0–3 scale: 0, negative; 1, weak; 2, intermediate; and 3, strong. The final score was designated as the sum of extent and intensity and samples categorized as negative (0), weak (1–2), moderate (3), and strong staining (4–6). For statistical calculations, moderate and strong staining scores were considered positive and other final scores considered negative.
Western immunoblotting
Total protein extracts from tissue were separated by 10% SDS-PAGE (20 mg per lane), then transferred onto a polyvinylidene fluoride membrane. Membranes were blocked and then probed with primary antibodies against CLIC1 (1:800 dilution; Abcam, Cambridge, UK) and GAPDH (1:500 dilution; Sino Biological, Beijing, China) at 4°C overnight. The secondary antibody (1:500 dilution; SAB, China) was placed under infrared light at room temperature for 1 hour and visualized using an enhanced chemiluminescence-detection reagent.
Real-time quantitative PCR
Total RNA was isolated from tissue using Trizol reagent (Takara, Kusatsu, Japan). The primers used in this study were shown in . RNA was reverse-transcribed into cDNA with a ReverTra Ace qRT-PCR kit (Takara, Japan). qRT PCR was performed on a StepOnePlus RT-PCR system (Thermo Fisher Scientific, Waltham, MA, USA). The reaction comprised an initial denaturation step of 95°C for 30 minutes, followed by 39 cycles of 95°C for 5 seconds, 60°C for 34 seconds, and a final extension step of 95°C for 10 minutes. The mRNA-expression level of the target gene in each sample was normalized to the GADPH control.
Table 2 Quantitative real-time PCR primers used in this study
ELISA
Blood samples in heparin tubes were centrifuged (2,000 g) at 4°C for 10 minutes to obtain plasma and frozen at −80°C for further analysis. CLIC1 protein levels in plasma were measured with human ELISA kits according to the manufacturer’s instructions. The results were processed with an automated analyzer (PerkinElmer Inc., Waltham, MA, USA).
Statistical analysis
Data were analyzed using SPSS 20.0. All data are expressed as means ± SD. Independent Student’s t-tests and χ2 tests were used to assess the effects of CLIC1 on tissue and plasma. The Kaplan–Meier test was used for univariate survival analysis. The Cox proportional-hazard model was used for multivariate analysis and for determining 95% CIs. Pearson correlation coefficients were used to assess correlations among tumor-associated genes. Results were regarded as significant at P<0.05.
Results
CLIC1 significantly upregulated in OSCC tissue
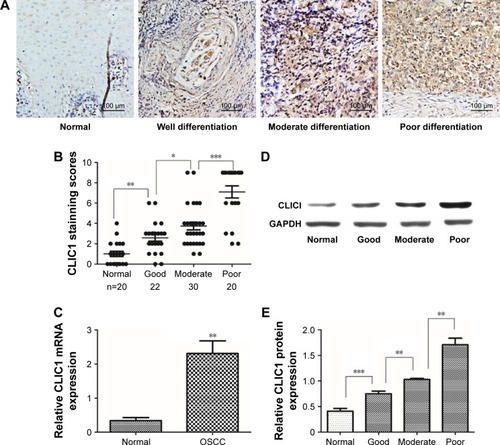
To evaluate CLIC1-expression status in human OSCC tissue, 26 pairs of OSCC and noncancerous oral tissue were collected to examine CLIC1 mRNA expression by qRT-PCR. Results showed that CLIC1 mRNA expression was upregulated in OSCC tissue (2.31±1.27) compared with noncancerous oral tissue (0.34±0.32, P=0.003; ). CLIC1 protein expression was higher in OSCC tissue (1.16±0.44) than paired noncancerous oral tissue (0.41±0.93) via Western blot, and this was consistent with qRT-PCR results. Furthermore, CLIC1 protein expression in different histological grades showed significant differences (P<0.001; ).
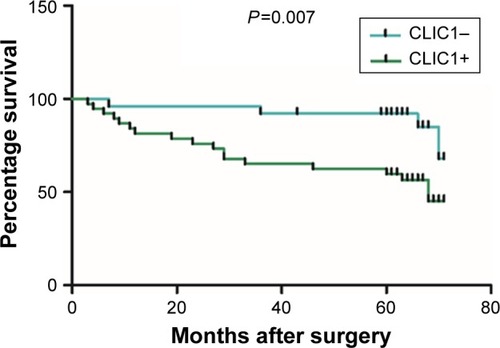
Figure 1 CLIC1 expression was significantly increased in oral squamous cell carcinoma (OSCC) tissue.
CLIC1 expression associated with clinicopathological features in OSCC patients
To elucidate the biological functions of CLIC1 in OSCC development, the expression of this protein in OSCC was compared with normal control tissue via IHC. Our results showed that CLIC1 is localized mainly in the nucleus and can also be observed in membranes and cytoplasm of cancer cells. Its protein expression differed in tumors of different differentiation (). Approximately 70.83% (51 of 72) of the OSCC patients displayed positive CLIC1-immunoreactive cells, while only 10.00% (two of 20) of the patients had positive immunoreactive cells in the normal control group (P<0.001; ). In addition, there were significant CLIC1 proteins of histological grade. Then, we evaluated correlations between CLIC1 expression and histopathological parameters in the OSCC patients. showed the CLIC1 overexpression remarkably related to histological grade (P=0.049), TNM stage (P=0.025), and tumor size (P=0.021), but not with age, sex, or lymph-node metastasis. These results indicated a potential role of CLIC1 expression in promoting incursionary OSCC phenotypes.
Table 3 Association of CLIC1 expression with the clinicopathological characteristics of oral squamous cell carcinoma
Evaluation of CLIC1 expression predicts poor prognosis in patients
Among the 72 OSCC patients, only 64 patients’ survival information was collected through phone-call follow-up. Histopathological subtype (P=0.001), TNM stage (P=0.046), and lymph-node metastasis (P<0.001) were significantly relevant to survival time by Kaplan–Meier survival analysis. Survival figures showed that the survival time of the CLIC1-positive patients was obviously lower than that of patients with CLIC1-negative expression (P=0.025; , ). To identify risk factors of prognosis precisely, univariate Cox regression analysis was used. These data revealed that CLIC1 expression, histological grade, and lymph-node metastasis were adversely correlated with postoperative survival time, suggesting that overexpression of CLIC1 is a risk factor for OSCC ().
Table 4 Univariate analysis of overall survival of oral squamous cell carcinoma patients
Table 5 Multivariate analysis of overall survival of oral squamous cell carcinoma patients
Determination of CLIC1 as a plasma OSCC biomarker
As CLIC1 was overexpressed in OSCC tissues, we speculated that CLIC1 could be detected in blood samples of OSCC patients. To determine this possibility, a sensitive ELISA was conducted to detect the expression of CLIC1 protein in plasma. Plasma samples of 30 OSCC patients without any treatment, 14 who had undergone resection only, ten who had undergone resection and chemotherapy, and 22 healthy controls were examined. CLIC1 protein expression was significantly higher in OSCC plasma than that of healthy controls (P=0.023), CLIC1 protein expression obviously lower in resection patients than those of OSCC patients (P=0.039), and CLIC1-expression levels in resection- + chemotherapy-treated patients aberrantly lower than in resection-only patients and healthy controls (P=0.002 and P=0.000, respectively; ). Moreover, CLIC1-expression level in OSCC patients’ plasma was also obviously higher in stage I–II than III–IV (P=0.002; ). However, there was no statistical difference between moderate differentiation and good differentiation (P=0.459; ).
Figure 3 Elevated CLIC1 protein levels in oral squamous cell carcinoma (OSCC) plasma samples via ELISA.

Correlation of CLIC1 and tumor-associated gene expression in OSCC tissue
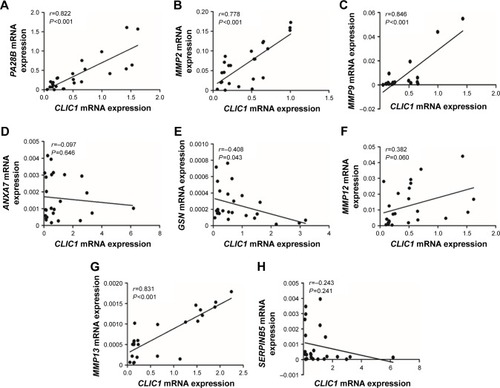
In order for profound understanding of CLIC1, tumor-associated genes were detected. In OSCC tissue, CLIC1 mRNA-expression level of was positively correlated with PA28B, MMP2, MMP9, MMP13, and MMP12 mRNA-expression levels, whereas CLIC1 mRNA expression level was negatively correlated with ANXA7, GSN, and SERPINB5 mRNA-expression levels (). Correlations between mRNA expression of GSN, PA28B, MMP2, MMP9, and MMP13 were significantly different from those of CLIC1 (P=0.043 and P<0.001).
Figure 4 Correlations between CLIC1 and PA28B, MMP2, MMP9, ANXA7, GSN, MMP12, MMP13, and SERPINB5 mRNA in OSCC according to qRT-PCR data.
Abbreviations: OSCC, oral squamous cell carcinoma; qRT-PCR, quantitative real-time PCR.
Discussion
In this research, our results suggested that CLIC1 plays an important role in the tumorigenic potential of human OSCC. CLIC1 mRNA and protein were overexpressed in OSCC tissue and CLIC1 protein closely associated with patients’ overall survival. Investigation indicated that CLIC1 is an independent metastasis and prognostic factor in OSCC. Moreover, CLIC1 protein was highly expressed in OSCC plasma and associated with therapy. Furthermore, our data demonstrated that CLIC1 may be involved in the pathway of development and metastasis of OSCC. Therefore, we consider CLIC1 has potential as a novel molecular therapeutic target and a possible outcome predictor.
CLIC1 has been reported to be upregulated in human malignant tumors of the colon, brain, liver, stomach, and lung.Citation14,Citation22,Citation24,Citation26,Citation27 It was evaluated to be the most penetrating receptor among commonly dysregulated proteins within three sarcomas.Citation23 This protein plays an important role in the metastasis of colonic cancer.Citation24 In gliomas, CLIC1 expression is inversely related to patient survival.Citation27 Moreover, CLIC1 participates in regulation of aggression in human lung adenocarcinoma.Citation26 Consistently with upregulated CLIC1 expression in a large number of tumors, our data suggested that CLIC1 mRNA and protein were highly expressed in OSCC tissue. Firstly, our study discovered that CLIC1 exists mainly in the nucleus, cytoplasm, and cell membranes of OSCC patients. Next, this research found that increased CLIC1 protein expression was relevant not only to tumor progression but also to poor patient survival, indicating its materiality in OSCC advancement. Notably, Cox multivariate analysis suggested that CLIC1-overexpression level was an independent prognostic factor for OSCC patients. Therefore, CLIC1 is a potential molecular therapeutic target for OSCC.
Increasing attention has been paid to blood research. CLIC1 has also been detected in the blood of patients, suggesting that CLIC1 could be useful as a blood marker. CLIC1 proteins secrete from tumor cells and can enter the circulation.Citation28 Chang et al first reported that there exists the possibility of CLIC1 as a plasma marker for nasopharyngeal carcinoma.Citation8 CLIC1 has been confirmed to be overexpressed in ovarian cancer patient serum.Citation22 It seems to have recently become a popular view that CLIC1 may be applied as general cancer screening markers. We detected CLIC1 protein in plasma samples of OSCC patients, and found that its expression level was significantly higher than in normal controls. CLIC1 protein expression in plasma was also correlated with advanced tumor status. Furthermore, it was closely associated with treatment. These results indicated that CLIC1 expression could be regarded as a biomarker and in indices for tumor metastasis and prognosis in OSCC. However, CLIC1 needs to be evaluated with further testing of larger patient cohorts to avoid false positives or false negatives before clinical application. In addition, because CLIC1 is associated with histological grade, TNM, and tumor size, but not with lymph-node metastasis, it may require coordination with other biomarkers for diagnosis and treatment of OSCC. CLIC1 could be included in a group of select biomarkers for early detection.
Although the potential role of CLIC1 in cancer is not clear, there are have been many advances. Some studies have reported that CLIC1 is linked to many tumor-associated proteins. Wei et al found that downregulation of CLIC1 increased maspin expression and reduced MMP2, MMP9, MMP12, and VEGF expression. Conversely, upregulation of CLIC1 decreased maspin expression and increased MMP2, MMP12, VEGF, and MMP13 expression.Citation29 Zhang et al provided the novel insight that CLIC1 has a vital role in hepatocarcinoma metastasis, perhaps via regulation of gelsolin and annexin A7 expression.Citation30 Other research reported that knockdown of PA28B increased tumor invasion and migration through upregulation of CLIC1.Citation31 What is more, CLIC1 may be correlated with the regulation of integrin-family proteins, which leads to subsequent regulation of the PI3K–AKT, MAPK–p38, and MAPK–ERK pathways.Citation14 To evaluate the biological function of CLIC1 in OSCC pathogenesis further, we compared CLIC1 with tumor-associated genes using PCR. We found that CLIC1 was relevant to a variety of tumor genes, especially PA28B, MMP2, and MMP13, whereas PA28B and matrix metalloproteinases (MMPs) played an important role in tumor metastasis and invasiveness.Citation31,Citation32 These data indicated that CLIC1 may be involved in the pathway of development and metastasis of OSCC. In vitro experiments and experiments on animals are required to elucidate the mechanism of CLIC1 acting in OSCC.
Conclusion
In summary, this study detected the expression of CLIC1 in OSCC tissue and plasma. The results pointed to CLIC1 perhaps being a novel biomarker for OSCC diagnosis, prognosis, and even early screening. The potential use of CLIC1 as an OSCC biomarker needs to be evaluated with further testing of larger patient cohorts and in-depth mechanical experiments.
Acknowledgments
This work was supported by funds provided by the Program for Innovation Team Building at the Institutions of Higher Education in Chongqing in 2016, Chongqing Municipal Key Laboratory of Oral Biomedical Engineering of Higher Education, Chongqing Research Program of Basic Research and Frontier Technology (PYZD201601), the Scientific and Technological Research Program of the Health and Family Planning Commission of Chongqing Province (2017ZDXM019 and 2016MSXM046), and Science and Technology Projects Foundation of Chongqing Yubei District Agricultural Bureau (2017-37).
Disclosure
The authors report no conflicts of interest in this work.
References
- ZhongLPZhangCPRenGXRandomized phase III trial of induction chemotherapy with docetaxel, cisplatin, and fluorouracil followed by surgery versus up-front surgery in locally advanced resectable oral squamous cell carcinomaJ Clin Oncol201331674475123129742
- WangCIYuCJHuangYAssociation of overexpressed karyopherin alpha 2 with poor survival and its contribution to interleukin-1β-induced matrix metalloproteinase expression in oral cancerHead Neck20184081719173329542209
- AquinoGFrancoRSabatinoRDeregulation of paralogous 13 HOX genes in oral squamous cell carcinomaAm J Cancer Res20155103042305526693058
- SuKJLinCWChenMKYangSFYuYLEffects of EZH2 promoter polymorphisms and methylation status on oral squamous cell carcinoma susceptibility and pathologyAm J Cancer Res20155113475348426807327
- SantiagoLDanielsGWangDDengFMLeePWnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatmentAm J Cancer Res2017761389140628670499
- HarropSJDeMaereMZFairlieWDCrystal structure of a soluble form of the intracellular chloride ion channel CLIC1 (NCC27) at 1.4-A resolutionJ Biol Chem200127648449934500011551966
- UlmasovBBrunoJWoostPGEdwardsJCTissue and subcellular distribution of CLIC1BMC Cell Biol20078817326840
- ChangYHWuCCChangKPYuJSChangYCLiaoPCCell secretome analysis using hollow fiber culture system leads to the discovery of CLIC1 protein as a novel plasma marker for nasopharyngeal carcinomaJ Proteome Res20098125465547419845400
- LuJDongQZhangBChloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancerMed Oncol201532661625920608
- KimJSChangJWYunHSChloride intracellular channel 1 identified using proteomic analysis plays an important role in the radiosensitivity of HEp-2 cells via reactive oxygen species productionProteomics201010142589260420461716
- WangPZengYLiuTChloride intracellular channel 1 regulates colon cancer cell migration and invasion through ROS/ERK pathwayWorld J Gastroenterol20142082071207824587680
- LiuYWangZLiMChloride intracellular channel 1 regulates the antineoplastic effects of metformin in gallbladder cancer cellsCancer Sci201710861240125228378944
- YeYYinMHuangBWangYLiXLouGCLIC1 a novel biomarker of intraperitoneal metastasis in serous epithelial ovarian cancerTumour Biol20153664175417925582317
- LiBPMaoYTWangZCLIC1 Promotes the Progression of Gastric Cancer by Regulating the MAPK/AKT PathwaysCell Physiol Biochem201846390792429669336
- NanawarePPRamtekeMPSomavarapuAKVenkatramanPDiscovery of multiple interacting partners of gankyrin, a proteasomal chaperone and an oncoprotein – evidence for a common hot spot site at the interface and its functional relevanceProteins20148271283130024338975
- HuangJSChaoCCSuTLDiverse cellular transformation capability of overexpressed genes in human hepatocellular carcinomaBiochem Biophys Res Commun2004315495095814985104
- HeYMZhangZLLiuQYEffect of CLIC1 gene silencing on proliferation, migration, invasion and apoptosis of human gallbladder cancer cellsJ Cell Mol Med20182252569257929516682
- SettiMOstiDRichichiCExtracellular vesicle-mediated transfer of CLIC1 protein is a novel mechanism for the regulation of glioblastoma growthOncotarget2015631314133142726429879
- QuHChenYCaoGIdentification and validation of differentially expressed proteins in epithelial ovarian cancers using quantitative proteomicsOncotarget2016750831878319927825122
- WangJWPengSYLiJTIdentification of metastasis-associated proteins involved in gallbladder carcinoma metastasis by proteomic analysis and functional exploration of chloride intracellular channel 1Cancer Lett20092811718119299076
- LomnytskaMIBeckerSGemollTImpact of genomic stability on protein expression in endometrioid endometrial cancerBr J Cancer201210671297130522415234
- TangHYBeerLATanyiJLZhangRLiuQSpeicherDWProtein isoform-specific validation defines multiple chloride intracellular channel and tropomyosin isoforms as serological biomarkers of ovarian cancerJ Proteomics20138916517823792823
- MurrayEHernychováLScigelovaMQuantitative proteomic profiling of pleomorphic human sarcoma identifies CLIC1 as a dominant pro-oncogenic receptor expressed in diverse sarcoma typesJ Proteome Res20141352543255924661138
- WangPZhangCYuPRegulation of colon cancer cell migration and invasion by CLIC1-mediated RVDMol Cell Biochem20123651–231332122426742
- JewellSDSrinivasanMMcCartLMAnalysis of the molecular quality of human tissues: an experience from the Cooperative Human Tissue NetworkAm J Clin Pathol2002118573374112428794
- WangWXuXWangWThe expression and clinical significance of CLIC1 and HSP27 in lung adenocarcinomaTumour Biol20113261199120821858536
- SettiMSavalliNOstiDFunctional role of CLIC1 ion channel in glioblastoma-derived stem/progenitor cellsJ Natl Cancer Inst2013105211644165524115360
- LittlerDRHarropSJGoodchildSCThe enigma of the CLIC proteins: Ion channels, redox proteins, enzymes, scaffolding proteins?FEBS Lett2010584102093210120085760
- WeiXLiJXieHChloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspinJ Gastroenterol Hepatol201530120821624989236
- ZhangJLiMSongMClic1 plays a role in mouse hepatocarcinoma via modulating Annexin A7 and Gelsolin in vitro and in vivoBiomed Pharmacother20156941641925661391
- ZhengDLHuangQLZhouFHuangQJLinJYLinXPA28β regulates cell invasion of gastric cancer via modulating the expression of chloride intracellular channel 1J Cell Biochem201211351537154622173998
- Vijaya KumarASalem GassarESpillmannDHS3ST2 modulates breast cancer cell invasiveness via MAP kinase- and Tcf4 (Tcf7l2)-dependent regulation of protease and cadherin expressionInt J Cancer2014135112579259224752740