Abstract
Background
Osimertinib is an EGFR-TKI that is selective for both EGFR-TKI-sensitizing and T790M resistance mutations in patients with non-small-cell lung cancer (NSCLC). The purpose of this study was conducting a meta-analysis to evaluate the clinical efficacy and safety of osimertinib in the treatment for NSCLC.
Methods
Using “osimertinib” as a keyword combined with “non-small-cell lung cancer” and “randomized controlled trial” as medical subject headings, the following electronic databases were searched: PubMed, EMBASE, Cochrane Library, and China National Knowledge Infrastructure. After data extraction and quality assessment of the included randomized controlled trials, the RevMan 5.3 software and R meta package were applied for meta-analysis of objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and safety.
Results
Ten studies met our criteria and were included in the meta-analysis, with a total of 3,260 participants. The meta-analysis showed that osimertinib therapy was superior to the control therapy alone in ORR (combined RR=1.53, 95% CI: 0.87–2.71, P=0.14), DCR (combined RR=1.07, 95% CI: 0.79–1.44, P=0.66), PFS (combined RR=0.32, 95% CI: 0.24–0.44, P<0.00001), and OS (combined RR=0.57, 95% CI: 0.47–0.70, P<0.00001). In addition, osimertinib led to some toxicities, and the overall prevalence of all-grade diarrhea was 40% (95% CI: 33–47), paronychia 26% (95% CI: 20–33), rash 40% (95% CI: 34–47), dry skin 28% (95% CI: 23–33), and stomatitis 15% (95% CI: 9–23).
Conclusion
Our study showed that osimertinib demonstrated a significant improvement in the ORR, DCR, PFS, and OS with tolerable adverse effects for NSCLC patients. However, because of some clear limitations (heterogeneity and publication bias), these results should be interpreted with caution.
Introduction
Among patients with locally advanced or metastatic non-small-cell lung cancer (NSCLC) with a mutant EGFR, the EGFR-TKIs such as gefitinib, erlotinib, and afatinib are recommended as the standard first-line therapy.Citation1,Citation2 Despite high initial tumor response rates to first-line EGFR-TKIs, most patients ultimately develop acquired resistance. Several common mechanisms of acquired resistance have been observed in recent studies, including EGFR Thr790Met resistance mutation, MET amplification, HER2 amplification, small-cell histological transformation, and epithelial to mesenchymal transition.Citation3,Citation4 It has been confirmed that the EGFR Thr790Met point mutation (EGFR T790M) can be detected in ≥50% of the patients taken after acquired resistance.Citation5
Until recently, there were several limitations on the treatment options in post-EGFR-TKI second-line setting, with low proportions of response to platinum-based doublet chemotherapy and monochemotherapy.Citation6 In addition, there was no global standard of care for the later-line therapy when patients experienced a failure of both EGFR-TKI therapy and platinum-based doublet chemotherapy; current treatment regimens for the same population are generally limited to monochemotherapy, rechallenge with the EGFR-TKIs, or experimental drugs in clinical trials.Citation7,Citation8
Osimertinib (Tagrisso, AZD9291; AstraZeneca plc) is an oral, potent, third-generation, irreversible EGFR-TKI that is selective for both EGFR-TKI-sensitizing and EGFR T790M resistance mutations, with a lower activity against wild-type EGFR. In previous studies, clinical activity and a manageable toxicity profile have been found in patients with T790M-positive NSCLC and acquired resistance to EGFR-TKIs.Citation9 It has been reported that osimertinib used as a second-line treatment has shown superior efficacy in NSCLC patients as compared with platinum chemotherapy in recent researches. On the basis of positive results from the clinical program, the US Food and Drug Administration (FDA) approved that osimertinib is worldwide for the treatment of patients with metastatic T790M-positive NSCLC, following progression during or after EGFR-TKI therapy.Citation10,Citation11
Currently, several clinical trials of osimertinib treated for NSCLC from Phase I to III have been published. However, the efficacy and safety information of these clinical studies are not identical. There has been no systematic attempt to synthesize the efficacy and safety data of this agent taking into consideration the fact that osimertinib is increasingly evaluated in NSCLC.Citation12 Therefore, the goal of our analysis was to evaluate the clinical efficacy (overall response rate, disease control rate [DCR], progression-free survival [PFS], and overall survival [OS] rate) and safety parameters (RR and incidence of all-grade adverse events [AEs]) to provide systematical clinical evidence for use of osimertinib.
Materials and methods
Search strategy
This meta-analysis followed the Cochrane Collaboration definition and PRISMA 2009 guidelines for meta-analysis and systematic review. We searched the electronic databases of PubMed, Excerpta Medica Database (EMBASE), Cochrane Library, China National Knowledge Infrastructure (CNKI), Chinese Scientific Journals Full-Text Database (CSJFT), Wanfang Data Knowledge Service Platform (WKSP), and the Chinese Biomedical Literature Service System (CBMdisc), and the time period for literature search was from the first available study until August 1, 2018. The keywords used in this search were as follows: “Osimertinib”, “EGFR-TKIs”, “non-small-cell lung cancer”, “NSCLC”, and “cancers” as well as “clinical trials”. In addition, we also searched the abstracts that contained “osimertinib in patients with cancers” presented at the European Society for Medical Oncology (ESMO) and major meetings from the American Society of Clinical Oncology (ASCO). Finally, the references lists of original articles and review articles from the Web of Science (WOS) database were also scanned to ensure that no additional studies were missed.
Study selection
Clinical trials that met the following criteria were included: 1) prospective Phase I, II, and III clinical trials of osimertinib treatment in the patients with NSCLC; 2) reporting the data on objective response rate (ORR), DCR, PFS, and OS, as well as AEs. Exclusion criteria were the following: 1) repeat studies, abstracts, letters, reviews, editorial, or comment and 2) published against the inclusion criteria. The PICO framework guiding the development of the search strategy is shown in .
Table 1 Eligibility criteria of the systematic review
Data extraction and quality assessment
Two reviewers (Peng Chen and Fuchao Chen) independently screened the titles and abstracts of each study. The following information from each study was extracted to understand the baseline of all included studies: the first author, year of publication, number of patients enrolled in the study, therapeutic regimen, and dose of the participants. To evaluate the methodological quality of the included literature, a modified Jadad scale was used to assess the quality of the included randomized studies. The scores of high-quality studies ranged from 4 to 8, whereas those of low-quality studies were from 0 to 3. For non-randomized studies, the quality was assessed using Newcastle–Ottawa Quality Assessment Scale. Each study was graded as either low quality (0–5) or high quality (6–9). Any disagreements were resolved by the third author.
Definition of main outcomes
The ORR was defined as a proportion of patients having a confirmed best response of complete response or partial response as assessed by the researchers. DCR is defined as the number of patients who had a best response rating of complete response, partial response, or stable disease. The definition of PFS was that the time between the date of randomization and the date of documented progression or death, whichever occurred first. OS was calculated as the time between the first dose of study treatment and date of death. The response was evaluated by the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 criteria, on the basis of assessment by an independent radiology review committee. For the safety analysis, we collected data about five frequent toxicity events, which included diarrhea, paronychia, rash, dry skin, and stomatitis. AEs were assessed using the National Cancer Institute Common Terminology Criteria (NCI CTC) version 4.0.
Statistical analyses
The differences between the osimertinib treatment and the control treatment were estimated by the pooled RR and HR along with 95% CIs. The summary RR and HR estimates were conducted using a random- or fixed-effect model. Between-study heterogeneity was evaluated by P-value and the I2 statistic. If I2 was <50% (Pheterogeneity>0.1), the fixed-effect model was used, if not, the random-effect model was performed. All calculations were performed by Review Manager Version 5.3 software (The Cochrane Collaboration, Oxford, UK) and R meta package (version 2.13.2) for Windows at 64 bits. P-values <0.05 or 0.01 were considered statistically significant.
Results
Search results
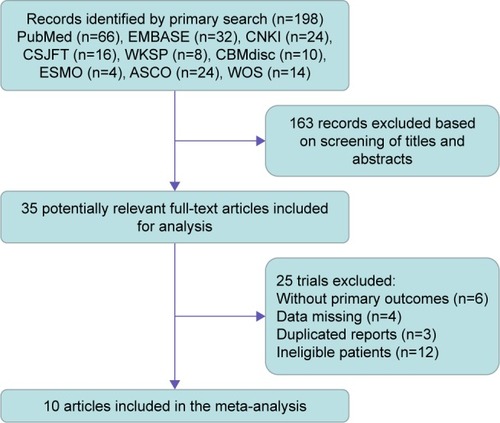
Overall, a total of 198 potential citations were identified according to the systematic literature searched for trials on osimertinib. Of the studies initially identified, we excluded reports that did not fulfill our inclusion criteria after first reading the titles and abstracts. Finally, our literature search yielded a total of 10 studies available for the meta-analysis, including two Phase I studies,Citation16,Citation18 two Phase I/II studies,Citation17,Citation22 one Phase II study,Citation14 and five Phase III studies.Citation13,Citation15,Citation19–Citation21 The flowchart describing the trial screening and selection procedure is shown in . Within the selected studies, there were five single-arm trails and five randomized control trials (RCTs), comprising a total of 3,260 patients. In all studies, the starting dose and schedule of osimertinib were based on US FDA guidelines (80 or 160 mg, orally, twice a day). The baseline characteristics of patients varied among trials, and the specific information of included trials is listed in .
Table 2 Baseline characteristics of trials included in the meta-analysis
Figure 1 Flow diagram of the study selection process.
Statistical analysis of efficacy outcomes
Comparison between osimertinib with controls alone
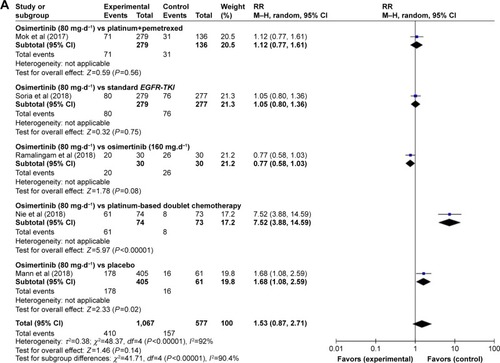
RR of ORR
The summary RR of ORR for osimertinib vs control treatment was 1.53 (95% CI: 0.87–2.71) using a random-effect model (heterogeneity: χ2=48.37, df=4 [P=0.64], I2=92%; ). As shown in , the ranking probabilities of comparison between osimertinib and control treatment from the subgroup analysis of ORR indicated that the highest RR of ORR was observed in patients associated with osimertinib vs platinum-based doublet chemotherapy (RR: 7.52 [95% CI: 3.88–14.59], P<0.00001), followed by osimertinib vs placebo (RR: 1.68 [95% CI: 1.08–2.59], P=0.02), osimertinib vs platinum combined pemetrexed (RR: 1.12 [95% CI: 0.77–1.61], P=0.56), osimertinib vs standard EGFR-TKI (RR: 1.05 [95% CI: 0.80–1.36], P=0.75), and osimertinib (80 mg⋅d−1) vs osimertinib (160 mg⋅d−1; RR: 0.77 [95% CI: 0.58–1.03], P=0.08).
Table 3 Outcomes of effectiveness for osimertinib in NSCLC patients
Figure 2 Forest plots analysis of the efficiency outcomes of osimertinib vs control treatment alone.
Abbreviations: ORR, objective response rate; PFS, progression-free survival; OS, overall survival; M–H, Mantel–Haenszel; SE, standard error; IV, intravenous.
RR of DCR
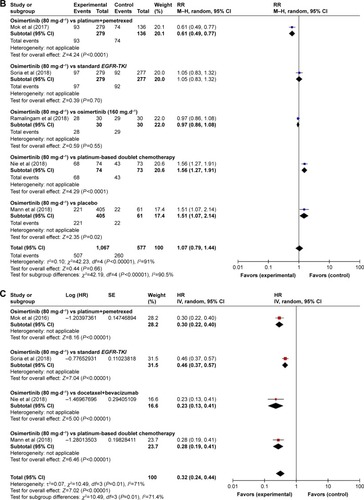
The RR of DCR was reported by five studies.Citation13,Citation15,Citation16,Citation20,Citation21 Obvious heterogeneity (χ2=42.23, df=4 [P<0.00001], I2=91%) was present among the included studies. The estimated RR of osimertinib vs control treatment by the random-effects model was 1.07 (95% CI: 0.79–1.44; ). For the subgroup analysis of RR of DCR, osimertinib vs platinum combined pemetrexed (RR: 0.61, 95% CI: 0.49–0.77, P<0.0001), osimertinib vs platinum-based doublet chemotherapy (RR: 1.56, 95% CI: 1.27–0.77, P<0.0001), and osimertinib vs placebo (RR: 1.51, 95% CI: 1.07–2.14, P=0.02) showed a significant difference, while osimertinib vs standard EGFR-TKI and osimertinib (80 mg⋅d−1) vs osimertinib (160 mg⋅d−1) showed no significant difference (RR: 1.05, 95% CI: 0.83–1.32, P=0.70; RR: 0.97, 95% CI: 0.86–1.08, P=0.55; ).
HR of PFS
Four studiesCitation13,Citation15,Citation19,Citation20 provided the information on PFS, and the HR values were explicitly reported in these studies. As shown in , the results of our random-effects (χ2=10.49, df=3 [P=0.01], I2=71%) meta-analysis for PFS indicated that there was a significant difference in HRs for osimertinib therapy vs control therapy (HR: 0.32 [95% CI: 0.24–0.44], P<0.00001), which indicated a 68% reduction in the risk of disease progression in patients treated with osimertinib-based method. The results of subgroup analysis showed that osimertinib significantly prolonged PFS as compared with combination of platinum and pemetrexed (HR: 0.30, 95% CI: 0.22–0.40, P<0.00001), standard EGFR-TKI (HR: 0.46, 95% CI: 0.37–0.57, P<0.00001), docetaxel combined bevacizumab (HR: 0.23, 95% CI: 0.13–0.41, P<0.00001), or platinum-based doublet chemotherapy (HR: 0.28, 95% CI: 0.19–0.41, P<0.00001) alone.
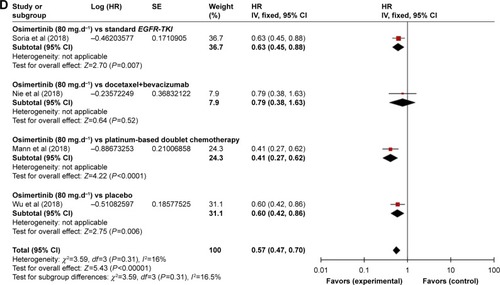
HR of OS
Total four RCTsCitation15,Citation19–Citation21 reported this outcome contributed to the analysis of OS. Heterogeneity between the four trials was χ2=3.59, df=3 (P<0.00001), I2=16%. After an analysis with fixed-effect model, we got the result that HR: 0.57 (95% CI: 0.47–0.70), P<0.00001 (). We also found that HR for OS in osimertinib vs standard EGFR-TKI, osimertinib vs docetaxel–bevacizumab, osimertinib vs platinum-based doublet chemotherapy, and osimertinib vs placebo were HR: 0.63 (95% CI: 0.45–0.88), P=0.007; HR: 0.79 (95% CI: 0.38–1.63), P=0.52; HR: 0.41 (95% CI: 0.27–0.62), P<0.0001; and HR: 0.60 (95% CI: 0.42–0.86), P=0.006, respectively.
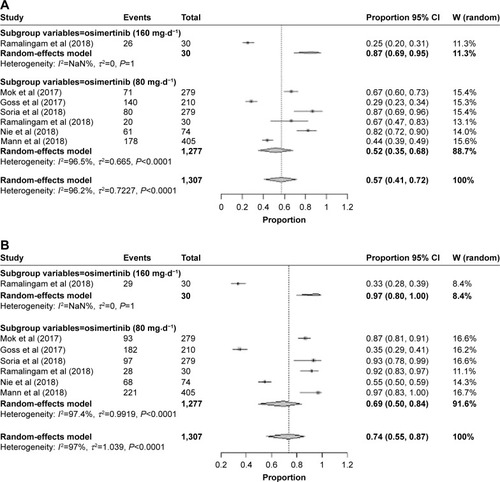
Pooled ORR and DCR
Results of the random-effect model (heterogeneity: I2=96%, P<0.0001) showed that the pooled ORR of the whole population of osimertinib was 0.57 (95% CI: 0.41–0.72; ). Six studiesCitation13–Citation16,Citation19,Citation20 presented the data about DCR; the pooled rate was 0.74 (95% CI: 0.56–0.87) with a significant heterogeneity (I2=97%, P<0.0001), which is shown in . A subgroup analysis has been conducted according the dosage of osimertinib treatment (80 and 160 mg⋅d−1). The results of the subgroup analysis showed that the pooled rate of ORR in osimertinib 80 mg⋅d−1 and osimertinib 160 mg⋅d−1 was 0.87 (95% CI: 0.69–0.95) and 0.52 (95% CI: 0.35–0.68), respectively, and DCR was 0.97 (95% CI: 0.80–1.00) and 0.69 (95% CI: 0.50–0.84), respectively.
Statistical analysis of safety outcomes
RR of all-grade AEs
A meta-analysis of the RR of all-grade AEs was performed on the included RCTs. Results of the random-effect or fixed-effect model showed that the pooled RR of all-grade AEs (diarrhea, paronychia, rash, dry skin, and stomatitis) with osimertinib therapy vs controls was 2.31 (95% CI: 0.72–7.42), 3.70 (95% CI: 0.19–72.70), 4.22 (95% CI: 0.40–44.62), 3.98 (95% CI: 0.67–23.47), and 1.22 (95% CI: 0.83–1.78), respectively (; ).
Table 4 Subgroup analysis of all-grade AEs for osimertinib in NSCLC patients
Figure 4 Subgroup analysis of the RR of all-grade AEs for osimertinib vs control treatment alone.
Abbreviations: AE, adverse event; M–H, Mantel–Haenszel.
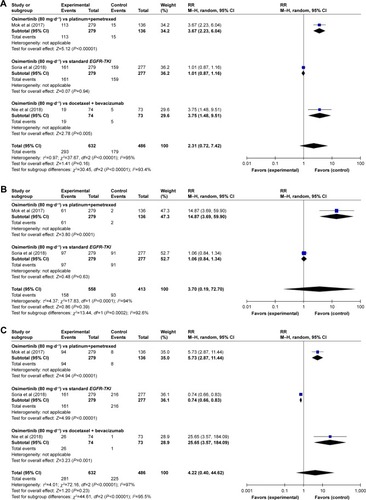
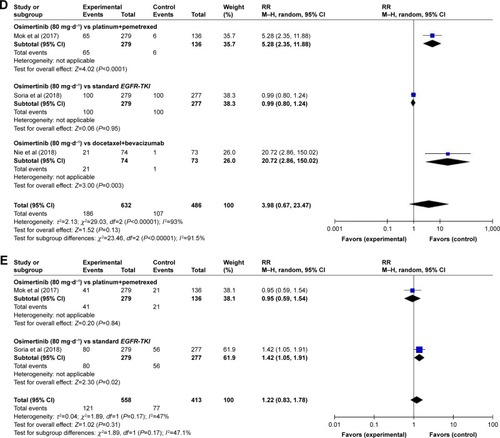
The subgroup analyses for the risk of all-grade AEs have been performed according to the type of treatment (osimertinib vs platinum+pemetrexed, osimertinib vs standard EGFR-TKI, osimertinib vs docetaxel+bevacizumab, osimertinib vs platinum-based doublet chemotherapy, and osimertinib vs placebo). By the subgroup analysis of the RR of all-grade AEs for osimertinib therapy vs controls, the following were found: there was a significant difference in RRs of all-grade diarrhea associated with osimertinib vs platinum combined pemetrexed and osimertinib vs docetaxel combined bevacizumab (RR=3.67, 95% CI: 2.23–6.04, P<0.00001; RR=3.75, 95% CI: 2.23–6.04, P=0.005), while no significant differences were observed in osimertinib vs standard EGFR-TKI (RR=1.01, 95% CI: 0.87–1.16, P=0.94; ).
As for paronychia, osimertinib vs platinum combined pemetrexed showed significant results (RR=14.87, 95% CI: 3.69–59.90, P=0.0001), whereas osimertinib vs standard EGFR-TKI showed no significant results (RR=1.06, 95% CI: 0.84–1.34, P=0.63; ). Regarding the rash, we found that the comparison between RR of high-grade rash events was more higher in osimertinib vs docetaxel combined bevacizumab (RR=25.65, 95% CI: 3.57–184.09, P<0.00001), followed by osimertinib vs platinum combined pemetrexed and osimertinib vs standard EGFR-TKI (RR=5.73, 95% CI: 2.87–11.44, P<0.00001 and RR=0.74, 95% CI: 0.66–0.83, P<0.00001, respectively; ).
Regarding the dry skin, the RR and 95% CI for dry skin in osimertinib vs platinum combined pemetrexed and osimertinib vs docetaxel combined bevacizumab were 5.28 (2.35–11.88) and 20.72 (2.86–150.02), respectively and resulted significantly (P<0.0001 and P=0.003, respectively). However, there were no significant differences between RR of all-grade dry skin in osimertinib vs standard EGFR-TKI (RR=0.99, 95% CI: 0.80–1.24, P=0.95; ). Finally, osimertinib vs standard EGFR-TKI showed a significant difference in all-grade stomatitis (RR=1.42, 95% CI: 1.05–1.91, P=0.02). No significant differences were observed between osimertinib vs platinum combined pemetrexed (RR=0.95, 95% CI: 0.59–1.54, P=0.84; ).
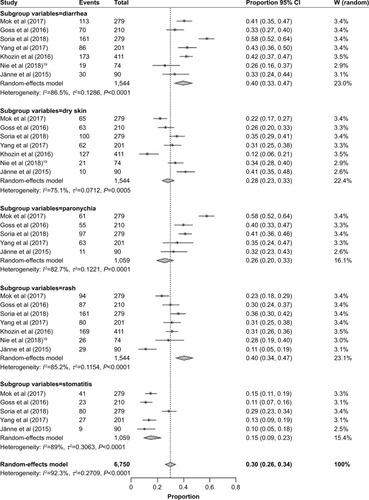
Incidence of all-grade AEs
In general, seven studiesCitation13–Citation15,Citation17–Citation19,Citation22 provided the data on incidence of all-grade AEs. Rates of the all-grade common AEs of osimertinib were analyzed and included diarrhea (40%, 95% CI: 33–47), paronychia (26%, 95% CI: 20–33), rash (40%, 95% CI: 34–47), dry skin (28%, 95% CI: 23–33), and stomatitis (15%, 95% CI: 9–23). More details are presented in .
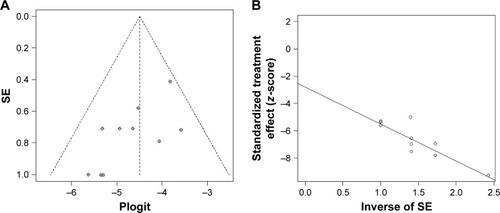
Publication bias
Publication bias was assessed using Egger’s funnel plot and Egger’s test in this study. The funnel plot and Egger’s funnel plot are displayed in . From funnel plot, it appeared a certain asymmetry, indicating that there is a certain degree of publication bias in the literature. However, the number of studies included is small, and thus, the funnel plot may not be convincing. Additionally, it was revealed that the publication bias was not significant according to the Egger’s test for the incidence of all-grade AEs (P=0.41).
Discussion
Prior to the approval of osimertinib, approaches to address patients with EGFR-T 790M mutation-positive NSCLC, the most common cause of acquired drug resistance in EGFRm NSCLC, have been limited by a lack of efficacy and dose-limiting toxicity. Osimertinib is currently supported in North America, Europe, and Asia as a recommendable treatment for patients with metastatic NSCLC who have progressed on EGFR-targeted therapy and whose tumors harbor a T790M mutation.Citation23 The approval was based on evidence from published randomized, open-label, international trials.Citation13–Citation22 Prior to summary of randomized, comparative control data for osimertinib, across different endpoints, from these studies, we performed a meta-analysis to evaluate the curative effectiveness and safety of osimertinib in the treatment to provide systematical clinical evidence for targeted therapy.Citation24,Citation25
Osimertinib is a recommended first-line treatment for patients with metastatic EGFR Thr790Met-positive NSCLC.Citation26 To our knowledge, this study is the first meta-analysis to report the data with a EGFR Thr790Met-directed EGFR-TKI.Citation27,Citation28 From our results, we found that patients with T790M-positive advanced NSCLC who received osimertinib had better ORR, DCR, PFS, and OS than did those receiving platinum therapy plus pemetrexed, standard EGFR-TKI, combination therapy of docetaxel with bevacizumab, and platinum-based doublet chemotherapy.Citation29 Interestingly, a subgroup analysis of pooled rate of objective response and disease control showed that superior effect was found in 160 mg osimertinib first-line treatment group than that of 80 mg group. Although the fact was observed, there are many studies that supported approved 80 mg once-daily dosage as the first-line therapy based on a comprehensive review of available safety, tolerability, efficacy, and pharmacokinetic data from first-and later-line patients treated with osimertinib.Citation30,Citation31 It has already been reported that a higher number of dose reductions as a result of AEs was observed in the 160 mg treatment group, which is consistent with available data from later-line patients treated with osimertinib.Citation32 Despite these advantages, osimertinib revealed some additional toxicities. Our safety results in this study were consistent with expectations from extensive previous reports.Citation33 The most common AEs possibly treatment related to osimertinib were rash (40%), diarrhea (40%), dry skin (28%), paronychia (26%), and stomatitis (15%). Based on our further subgroup analysis of risk of AEs, it is not difficult to find that the regimen of osimertinib (80 mg·d−1) carries a lower risk in paronychia and rash compared to the standard EGFR-TKI therapy. Moreover, the subgroup analysis also showed that a more higher risk was in osimertinib vs docetaxel combined bevacizumab for diarrhea, rash, and stomatitis when compared with osimertinib vs platinum combined pemetrexed.
Mechanisms of resistance to treatment with early-generation EGFR-TKIs when they are used as the first-line therapy have been described previously, with EGFR T790M being the most common resistance mutation; other resistance mechanisms that have been reported include amplification of HER2, MET, and MAPK1; mutation of PIK3CA and BRAF; and small-cell transformation.Citation34 Mechanisms of resistance to osimertinib that have also been identified in patients include KRAS amplification and acquired EGFR C797S mutation.Citation35 Consistent with preclinical data and its mechanism of action, mechanisms of resistance to osimertinib when used as the first-line therapy remain to be fully characterized, although the result from a Phase I study showed that initial treatment with osimertinib did not result in emergence of T790M as the mechanism of drug resistance, as assessed using ctDNA from plasma samples at or after clinical progression.Citation36 Nine patients had putative genomic resistance mechanisms identified. Two instances of acquired C797S were identified, one in absence of a T790M mutation. This finding has potentially important clinical implications, because quinazoline-based EGFR inhibitors, including gefitinib, have been shown to effectively inhibit C797S when T790M is absent.Citation37,Citation38 In the report by Ramalingam et al, they identified EGFRm in 10 patients but no putative resistance mechanism at the time of progression. It is possible that molecular changes only detectable at the tissue level (eg, NSCLC transformation) and any nongenomic mechanisms of resistance were not identified in this analysis.Citation16 It has been suggested that tissue-based analyses of resistance mechanisms will be considered as a approach to fully characterize resistance to osimertinib, and the analysis of ctDNA from identified post-progression plasma samples had involved either activation of pathways downstream of EGFR (MAPK pathway signaling) or activation of parallel signaling pathways (MET and HER2), providing the possibility of combination approaches after progression on the first-line osimertinib therapy.Citation39,Citation40
For the AEs associated with osimertinib, it was generally manageable with established treatment guidelines.Citation41 Generally speaking, first, patients should be advised about the importance of managing AEs at an early stage, and the health care team of NSCLC patients should be informed to be ready for nutrition to avoid hyponatremia or hypokalemia when the gastrointestinal events, which is the most frequent AEs associated with osimertinib, occur.Citation42 Second, it is suggested to strictly take action of dosage reduction (40 mg⋅d−1) when grade 2 events occurred, and there may be a need to permanently discontinue medication at the onset of grade 3 or higher events.Citation43 Third, the duration and dosage of ipilimumab or nivolumab should be based on the severity of the patient’s underlying disease, recovery from immunosuppression, and clinical response.Citation44 Moreover, in view of pharmacoeconomics, osimertinib is not covered by health insurance, and patients have to pay for the expenses of taking them all on their own.Citation45 The average daily cost of osimertinib for adult patients is approximately $249.6438 (at a dose of 80 mg once daily, 80 mg×30 pills/box [TAGRISSO] for a month). However, further studies would be required to confirm these derived conclusions.Citation46
Limitations
The current research also had some limitations, which need to be addressed. First and most important, the number of studies and patients included in this study is small and there was a lack of sufficient data and sample size to be reliable. Second, we did not perform subgroup analysis of high-grade events because of lack of enough information. Third, the heterogeneity among the results of the studies was evident, which significantly decreased the statistical power of the analysis. Furthermore, on the basis of our study, the efficacy and safety of osimertinib combined with other therapy were unknown. Finally, the publication bias might have been occurred, and it could not be completely reflected by funnel plot. Therefore, future additional high-quality, large-sample, multicenter, randomized controlled clinical trials are needed to resolve these limitations.
Conclusion
Based on the results of current meta-analysis, osimertinib, a molecularly targeted single agent, is favorable to improve the survival outcomes, including the objective response, DCRs, PFS, and OS, although it may increase the incidence of some AEs. In addition, correct estimates of treatment-related toxicities and the efficacy of osimertinib could be fundamental to provide appropriate guidance and conduct ongoing trials. Further RCTs were warranted to update our meta-analysis and investigate the role of osimertinib in first line for NSCLC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
- SunCLiSYangCMicroRNA-187-3p mitigates non-small cell lung cancer (NSCLC) development through down-regulation of BCL6Biochem Biophys Res Commun20164711828826845350
- ReckampKLMelnikovaVOKarlovichCA highly sensitive and quantitative test platform for detection of NSCLC EGFR mutations in urine and plasmaJ Thorac Oncol201611101690170027468937
- DongJKLeiHMLiangQOvercoming erlotinib resistance in EGFR mutation-positive lung adenocarcinomas through repression of phosphoglycerate dehydrogenaseTheranostics2018871808182329556358
- LinCCShihJYYuCJOutcomes in patients with non-small-cell lung cancer and acquired Thr790Met mutation treated with osimertinib: a genomic studyLancet Respir Med20186210711629249325
- JakobsenJNSantoni-RugiuEGrauslundMMelchiorLSørensenJBConcomitant driver mutations in advanced EGFR-mutated non-small-cell lung cancer and their impact on erlotinib treatmentOncotarget2018940261952620829899852
- XuJZhangYJinBEfficacy of EGFR tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutations in ChinaJ Cancer Res Clin Oncol201614261325133026942444
- DaiDLiXFWangJPredictive efficacy of (11)C-PD153035 PET imaging for EGFR-tyrosine kinase inhibitor sensitivity in non-small cell lung cancer patientsInt J Cancer201613841003101226334931
- InoueAYoshidaKMoritaSCharacteristics and overall survival of EGFR mutation-positive non-small cell lung cancer treated with EGFR tyrosine kinase inhibitors: a retrospective analysis for 1660 Japanese patientsJpn J Clin Oncol201646546246726977054
- ZhangCGYinDDSunSYHanLThe use of lncRNA analysis for stratification management of prognostic risk in patients with NSCLCEur Rev Med Pharmacol Sci201721111511928121347
- SchoenfeldJDSibenallerZAMapuskarKAO2⋅− and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbateCancer Cell201731448750028366679
- BallardPYatesJWYangZPreclinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activityClin Cancer Res201622205130514027435396
- ZhaoJQiaoCRDingZA novel pathway in NSCLC cells: miR-191, targeting NFIA, is induced by chronic hypoxia, and promotes cell proliferation and migrationMol Med Rep20171531319132528075452
- MokTSWuY-LAhnM-JOsimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancerN Engl J Med2017376762964027959700
- GossGTsaiCMShepherdFAOsimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (AURA2): a multicentre, open-label, single-arm, phase 2 studyLancet Oncol201617121643165227751847
- SoriaJCOheYVansteenkisteJOsimertinib in untreated EGFR-mutated advanced non-small-cell lung cancerN Engl J Med2018378211312529151359
- RamalingamSSYangJCLeeCKOsimertinib as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancerJ Clin Oncol201836984184928841389
- YangJCAhnMJKimDWOsimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA study Phase II extension componentJ Clin Oncol201735121288129628221867
- KhozinSWeinstockCBlumenthalGMOsimertinib for the treatment of metastatic epidermal growth factor T970M positive non-small cell lung cancerJ Cancer Res Clin201623921312135
- NieKZhangZZhangCOsimertinib compared docetaxel-bevacizumab as third-line treatment in EGFR T790M mutated non-small-cell lung cancerLung Cancer201812151129858027
- MannHAndersohnFBodnarCAdjusted indirect comparison using propensity score matching of osimertinib to platinum-based doublet chemotherapy in patients with EGFRm T790M NSCLC who have progressed after EGFR-TKIClin Drug Investig2018384319331
- WuYLHerbstRSMannHRukazenkovYMarottiMTsuboiMADAURA: Phase III, double-blind, randomized study of osimertinib versus placebo in EGFR mutation-positive early-stage NSCLC after complete surgical resectionClin Lung Cancer201819442294242
- JännePAYangJCKimDWAZD9291 in EGFR inhibitor-resistant non-small-cell lung cancerN Engl J Med2015372181689169925923549
- DouillardJYOstorosGCoboMFirst-line gefitinib in Caucasian EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm studyBr J Cancer20141101556224263064
- BhattVRD’SouzaSPSmithLMEpidermal growth factor receptor mutational status and brain metastases in non-small-cell lung cancerJ Glob Oncol20173320821728717762
- JenkinsSChih-Hsin YangJJännePAEGFR mutation analysis for prospective patient selection in two Phase II registration studies of osimertinibJ Thorac Oncol20171281247125628527899
- BertranouEBodnarCDanskVCost-effectiveness of osimertinib in the UK for advanced EGFR-T790M non-small cell lung cancerJ Med Econ201721211728881157
- JenkinsSYangJCRamalingamSSPlasma ctDNA analysis for detection of the EGFR T790M mutation in patients with advanced non-small cell lung cancerJ Thorac Oncol20171271061107028428148
- DevineAMarignolLPotential of amifostine for chemoradiotherapy and radiotherapy-associated toxicity reduction in advanced NSCLC: a meta-analysisAnticancer Res201636151226722022
- LöserPMrossBLappHSchwere Unverträglichkeitsreaktion unter Crizotinib bei ALK-positivem, metasiertem NSCLCPneumol20167012826830
- MamesayaNKenmotsuHKatsumataMNakajimaTEndoMTakahashiTOsimertinib-induced interstitial lung disease after treatment with anti-PD1 antibodyInvest New Drugs201735110510727599705
- ArulanandaSdoHMusaferAMitchellPDobrovicAJohnTCombination osimertinib and gefitinib in C797S and T790M EGFR-mutated non-small cell lung cancerJ Thorac Oncol201712111728173228843359
- RicciutiBBaglivoSPaglialungaLOsimertinib in patients with advanced epidermal growth factor receptor T790M mutation-positive non-small cell lung cancer: rationale, evidence and place in therapyTher Adv Med Oncol20179638740428607578
- MccoachCEJimenoAOsimertinib, a third-generation tyrosine kinase inhibitor targeting non-small cell lung cancer with EGFR T790M mutationsDrugs Today2016521056156827910964
- KotakeMMurakamiHKenmotsuHHigh incidence of interstitial lung disease following practical use of osimertinib in patients who had undergone immediate prior nivolumab therapyAnn Oncol2016283669670
- ChenKZhouFShenWNovel mutations on EGFR Leu792 potentially correlate to acquired resistance to osimertinib in advanced NSCLCJ Thorac Oncol2017126e65e6828093244
- ChicNMayo-de-Las-CasasCReguartNSuccessful treatment with gefitinib in advanced non-small cell lung cancer after acquired resistance to osimertinibJ Thorac Oncol2017126e78e8028532569
- NiederstMJHuHMulveyHEThe allelic context of the C797S mutation acquired upon treatment with third-generation EGFR inhibitors impacts sensitivity to subsequent treatment strategiesClin Cancer Res201521173924393325964297
- ErcanDChoiHGYunCHEGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitorsClin Cancer Res201521173913392325948633
- La MonicaSCretellaDBonelliMTrastuzumab emtansine delays and overcomes resistance to the third-generation EGFR-TKI osimertinib in NSCLC EGFR mutated cell linesJ Exp Clin Cancer Res201736117418529202823
- IamsWChaeYP3.02-034 Acquired resistance to osimertinib by CCDC6-RET fusion in a patient with EGFR T790M mutant metastatic lung adenocarcinomaJ Thorac Oncol20171211S2249S2250
- Avilés-SalasAMuñiz-HernándezSMaldonado-MartínezHAReproducibility of the EGFR immunohistochemistry scores for tumor samples from patients with advanced non-small cell lung cancerOncol Lett201713291292028356978
- ShawATGandhiLGadgeelSAlectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trialLancet Oncol201617223424226708155
- TanimotoATakeuchiSAraiSHistone deacetylase 3 inhibition overcomes BIM deletion polymorphism-mediated osimertinib resistance in EGFR-mutant lung cancerClin Cancer Res201723123139314927986747
- CallegariDRanaghanKEWoodsCJL718Q mutant EGFR escapes covalent inhibition by stabilizing a non-reactive conformation of the lung cancer drug osimertinibChem Sci20189102740274929732058
- HoyleCDyerMComments on cost-effectiveness of osimertinib for EGFR mutation-positive non-small-cell lung cancer after progression during first-line EGFR tyrosine kinase inhibitor therapyJ Thorac Oncol2018135e83e8429703545
- WuBGuXZhangQCost-Effectiveness of osimertinib for EGFR mutation-positive non-small cell lung cancer after progression following first-line EGFR TKI therapyJ Thorac Oncol201813218419329101057