Abstract
Primary clear cell carcinoma of pancreas is extremely rare. We present a case of a 64-year-old male with a mass in the distal body and tail of the pancreas. He underwent a distal pancreatectomy. The histopathology of tumor cells showed features with abundant clear cytoplasm and prominent cell boundaries. Immunohistochemical analysis of neoplastic cells showed reactions to antibodies against cytokeratin-7 and showed no reactions to antibodies against hepatocyte nuclear factor-1β, carbonic anhydrase 9, synaptophysin, and chromogranin A. The patient was subsequently diagnosed with a primary clear cell carcinoma of the pancreas. This is the first time we have encountered it. We report this rare case and update the current literature of this tumor.
Keywords:
Introduction
According to the WHO classification, primary clear cell carcinoma of the pancreas is classified as a rare “miscellaneous” carcinoma;Citation1 morphologically it is similar to the clear cell carcinoma of renal. It was first described by Cubilla in 1980.Citation2 Because only few cases have been reported in the literature, the systematic overview of this tumor entity is insufficient. We report a rare case of primary clear cell carcinoma of the pancreas, review, and update the medical literature reported of this neoplasm.
Case report
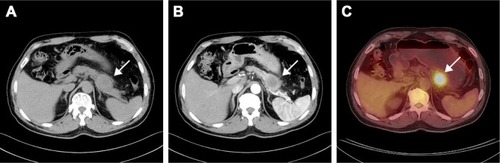
A 64-year-old male presented to our hospital with a year history of epigastric pain, radiating to the back, and 6 kg weight loss. He had no history of carcinoma and had a history of laparoscopic cholecystectomy 10 years ago. He had smoked 20 cigarettes per day for 30 years and drank alcohol on occasion. The family history was negative for malignant diseases. Physical examination revealed tenderness over the epigastric area. There were no enlarged superficial lymph nodes nor any palpable mass in the abdomen; the liver and spleen were not enlarged. The laboratory findings were all within the normal ranges except tumor markers. Tumor marker assay showed that serum carcinoembryonic antigen (CEA) was 18.56 ng/mL (normal: 0–5 ng/mL), the serum cancer antigen (CA) 19–9 was 649.15 U/mL (normal: 0–37 U/mL), CA125 was 132.97 U/L (normal: 0–35 kU/L), CA242 was 393.93 U/L (normal: 0–20 kU/L), neuron-specific enolase was 11 ng/mL (normal: 0–25 ng/mL), CYFRA21–1 was 1.7 ng/L (normal: 0–5 ng/L). Abdominal computed tomography (CT) showed a 40 × 45 mm heterogeneous irregular hypodense mass in the distal body and tail of the pancreas (), and it was enhanced after infusion of contrast material (). Positron emission tomography-CT showed diffuse abnormal uptake only in the pancreatic mass ().
Figure 1 CT showed a 40 × 45 mm heterogeneous irregular hypodense mass in the distal body and tail of the pancreas (A), and it was enhanced after infusion of contrast material (B). PET-CT showed diffuse abnormal uptake only in the pancreatic mass (C).
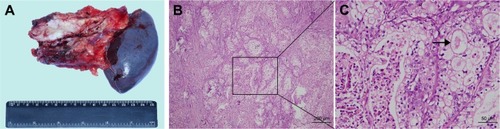
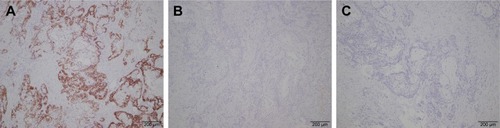
Preoperatively, it was diagnosed as an adenocarcinoma of the pancreas. Distal pancreatectomy and splenectomy were performed, and the specimen was sent for histopathological examination (). Microscopically, highly atypical glands were composed of round or oval bulky cells with abundant clear cytoplasm and well-defined cell borders (). These tumor cells were arranged as solid trabeculae, cords, and tubules. The hobnail appearance of cell arrangement was seen in some tumor cells. Nuclei were small, moderately pleomorphic with irregular borders, and often were eccentrically placed. These features were present in more than 90% of the tumor cells. Immunohistochemistry showed strong and diffuse staining for cytokeratin-7 (CK-7) (), and the immunohistochemistry staining was negative for chromogranin A (CGA), synaptophysin (SYN), hepatocyte nuclear factor-1β (HNF-1β) (), and carbonic anhydrase 9 (CA9) (). The patient recovered well and was discharged 10 days after surgery. CT scan revealed multiple hepatic metastasis 3 months after surgery, then he received gemcitabine chemotherapy and died 2 months later.
Discussion
Clear cell carcinoma occurs mostly in the kidney, lung, ovary, and thyroid, but this tumor extremely rarely originates from the pancreas.Citation3 This is also the first time we have encountered primary pancreatic clear cell carcinoma, diagnosed by pathology and immunohistochemistry. We summarized our case and the available 12 cases of pancreatic clear cell carcinoma reported previously (). The age of the patients (nine males and three females) was 46–75 years, an average age of 64.4 years. The clinical symptoms are not specific, mainly manifested as abdominal pain, radiating to the back, jaundice, anorexia weight loss, and so on. Tumor biomarkers in these patients, such as serum CA19–9, CA125, CA242, CEA, can be elevated or normal. There are no specific diagnostic criteria for it. The diagnosis relies mainly on typical pathological features with abundant clear cytoplasm and prominent cell boundaries. Immunohistochemistry is a complementary method to distinguish from other tumors. Immunohistochemistry almost showed reactions to antibodies against CK-7 in all cases, while showed no reactions to neuroendocrine markers such as CGA, SYN in all cases. Sequencing of the K-ras oncogene was analyzed in three cases,Citation4–Citation6 and a mutation at codon 12 in K-ras oncogene was detected in two cases,Citation5,Citation6 providing molecular evidence of ductal origin. Based on the above characteristics, it was believed that pancreatic clear cell carcinoma is a variation of pancreatic ductal adenocarcinoma.Citation5 HNF-1β may be a useful marker to identify these clear cell carcinomas, and its overexpression may aid in stratifying survival rate,Citation7 but in our case, HNF-1β was negative. Primary pancreatic clear cell carcinoma must be distinguished from the pancreatic neuroendocrine tumor, solid pseudopapillary tumor, mixed ductal-endocrine carcinoma, and pancreatic metastasis from clear cell renal cell carcinoma.Citation8 Of these, the most confusing tumor seems to be metastatic renal cell carcinoma.Citation9,Citation10 However, it is not very difficult to distinguish this tumor from it. History of renal tumors, preoperative CT, and MRI findings contribute to differential diagnosis. Postoperative immunohistochemistry of renal clear cell carcinoma showed positive reactions to antibodies against CD10, CA9, and Pax2, while immunonegative to CK-7.Citation11–Citation13 Because it is a rare tumor with very few cases reported previously, the incidence and prognosis are not well known for this neoplasm. However, we found that most of the reported cases showed aggressive behavior portending poor outcome, especially when the tumor was located in the tail of the pancreas.
Table 1 The previously reported cases of primary clear cell adenocarcinoma of the pancreas
Conclusion
We presented an extremely rare case of pancreatic clear cell ductal adenocarcinoma, which can be regarded as a rare variant of ductal adenocarcinoma in the proper morphology context supported by immunohistochemistry. Genetic analysis for detecting K-ras mutations and biomarker studies, such as HNF-1β, would aid in the identification of this rare neoplasm, but it is not absolute.
Acknowledgments
Written informed consent was acquired from the patient with approval of case detail and related patient information for publication use. Institutional approval was not required to publish this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
- HamiltonSRAaltonenLAWorld Health Organization classification of tumoursPathology and Genetics of Tumours of the Digestive SystemLyon, FranceIARC Press2000221250
- CubillaALFitzgeraldPJCancer (non-endocrine) of the pancreas. A suggested classificationMonogr Pathol198021821106261124
- ModiYShaabanHGauchanDMaroulesMParikhNGuronGPrimary clear cell ductal adenocarcinoma of the pancreas: a case report and clinicopathologic literature reviewJ Cancer Res Ther201410377377625313783
- SasakiAIshioTBandohTClear cell carcinoma of the pancreas: an adenocarcinoma with unusual phenotype of duct cell originJ Hepatobiliary Pancreat Surg200411214014415127279
- LüttgesJVogelIMenkeMHenne-BrunsDKremerBKlöppelGClear cell carcinoma of the pancreas: an adenocarcinoma with ductal phenotypeHistopathology19983254444489639120
- RaySLuZRajendiranSClear cell ductal adenocarcinoma of pancreas: a case report and review of the literatureArch Pathol Lab Med2004128669369615163226
- KimLLiaoJZhangMClear cell carcinoma of the pancreas: histopathologic features and a unique biomarker: hepatocyte nuclear factor-1betaMod Pathol20082191075108318536653
- LeeHYLeeDGChunKLeeSSongSYClear cell carcinoma of the pancreas – a case report and review of the literatureCancer Res Treat200941317518119809568
- BenhaimROussoultzoglouESaeediYMouracadePBachellierPLangHPancreatic metastasis from clear cell renal cell carcinoma: outcome of an aggressive approachUrology201585113514025530375
- HoshinoYShinozakiHKimuraYPancreatic metastases from renal cell carcinoma: a case report and literature review of the clinical and radiological characteristicsWorld J Surg Oncol20131128924209713
- TanPHChengLRioux-LeclercqNRenal tumors: diagnostic and prognostic biomarkersAm J Surg Pathol201337101518153124025522
- ZianneMTakahashiNTsujibataAMiwaKGotoYMatanoYAsymptomatic pancreatic metastasis from renal cell carcinoma diagnosed 21 years after nephrectomyCase Rep Gastrointest Med2017201716
- PickeringLMLarkinJKidney cancer: carbonic anhydrase IX in resected clear cell RCCNat Rev Urol201512630931026032554