Abstract
Cervical cancer patients who develop distant metastasis are rarely curable with very limited treatment options. Chemotherapy is often administered but with limited efficacy. Immunotherapy and anti-angiogenesis therapy are recommended for selected cases of recurrent or metastatic cervical cancers. The clinical efficacy of inhibitors targeting HER2, a commonly mutated gene in cervical cancer, has not been elucidated. Herein, we report a metastatic cervical adenocarcinoma patient carrying HER2 G292R who benefited from pyrotinib after progression on radio-chemotherapy, achieving complete response (CR) with a progression-free survival of 25 months and counting. Our study sheds light on the treatment options for previously treated metastatic cervical adenocarcinoma patients harboring activating HER2 mutations.
Introduction
Cervical cancer is one of the leading causes of morbidity and cancer deaths in women worldwide and in China.Citation1,Citation2 Local radiotherapy, chemotherapy or the combination of chemotherapy and bevacizumab are the standard first-line treatment for recurrent and metastatic cervical cancer,Citation3 but the current options for second-line and beyond treatment of cervical cancer remain limited.
HER2 (ERBB2) encodes the epidermal growth factor family member of receptor tyrosine kinases (RTKs). Somatic HER2 alterations including HER2 single nucleotide variants and HER2 amplification, occurring in 14% of cervical cancers, are considered to be oncogenic and associated with poor prognosis.Citation4 Pyrotinib, an irreversible pan-HER tyrosine kinase inhibitor against EGFR, HER2 and HER4, combined with capecitabine has been approved by China National Medical Products Administration for the treatment of patients with HER2-overexpressing advanced or metastatic breast cancer who have been previously treated with anthracycline or taxane chemotherapy.Citation5 Whether patients with HER2-mutant cervical cancer benefit from pyrotinib treatment has not been documented. In this study, we report the first clinical evidence of the efficacy of pyrotinib in a poorly differentiated metastatic cervical adenocarcinoma patient carrying HER2 G292R who progressed on radio-chemotherapy.
Case Presentation
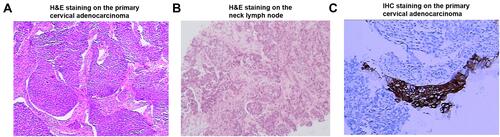
A woman in her 40s presented with irregular vaginal bleeding and was referred to our clinic in May 2018. The patient’s treatment history is shown in . She was diagnosed with stage IVB cervical adenocarcinoma (the red arrow in ) with metastases to parametrial lymph node, retroperitoneal lymph node, pelvic lymph node, and neck lymph node. Hematoxylin & eosin (H&E) staining showed a primary cervical carcinoma () and the metastases to neck lymph node (). Immunohistochemistry staining was performed on the primary cervical tumor and demonstrated that 1% of tumor cells were positive for HER2 overexpression (). The patient refused to receive chemotherapy at the beginning of treatment and instead received intensity-modulated radiation therapy (IMRT) on pelvis. Unfortunately, she developed metastases to liver (the red arrow in ) and left supraclavicular lymph node two weeks later. Under the consent of the patient, IMRT directed to neck in combination with brachytherapy and platinum-based chemotherapy (paclitaxel and nedaplatin) were administered in July 2018. The pelvic magnetic resonance imaging (MRI), abdominal MRI, and chest computed tomography (CT) scans in September 2018 demonstrated partial response (PR) in parametrial lymph node, retroperitoneal lymph node, pelvic lymph node, and metastatic liver tumor (the red arrow in ). Re-examination in December 2018 demonstrated the complete remission of the primary cervical tumor. The tumor assessment remained PR in metastatic liver tumor. In February 2019, the patient experienced disease progression with the newly developed metastatic liver lesion (the red arrow in ). The patient was subsequently treated with oral pyrotinib (400 mg/day) based on the presence of HER2 G292R with an allele frequency of 11.49%, negative HER2 amplification, and microsatellite stable (MSS) in cervical biopsy detected by capture-based targeted sequencing (Burning Rock Biotech, Guangzhou, China) using a panel consisting of 520 cancer-related genes prior to pyrotinib treatment. Due to the intolerant hand-foot syndrome as the pyotinib-related adverse event, the dose of pyrotinib was reduced to 240 mg/day. The patient achieved stable disease in May 2019 () and the complete response in the metastatic liver tumor (the red arrow in ) and metastases to lymph nodes in July 2020. She remained as tumor-free for 8 months and counting at the time of manuscript submission.
Figure 1 A summary of patient’s treatment history.
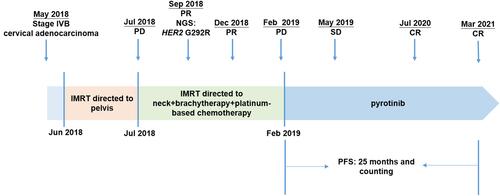
Figure 2 Magnetic resonance imaging (MRI) of the liver (upper) and cervical (lower) lesions at treatment milestones. (A) at baseline; (B) two weeks after IMRT directed to pelvis; (C) two months after IMRT directed to neck combined with brachytherapy and platinum-based chemotherapy (PR); (D) seven months after IMRT directed to neck combined with brachytherapy and platinum-based chemotherapy (PD). (E) three months after pyrotinib treatment (SD); (F) seventeen months after pyrotinib treatment (CR). The red arrows in upper and lower panels indicated metastatic live lesion and the primary cervical adenocarcinoma, respectively.
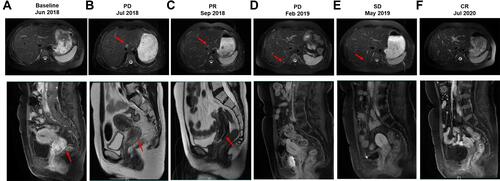
Figure 3 Hematoxylin & eosin (H&E)/immunohistochemistry (IHC) staining on the primary cervical adenocarcinoma/neck lymph node. (A) H&E staining on the primary cervical adenocarcinoma; (B) H&E staining on the neck lymph node; (C) IHC staining for HER2 on the primary cervical adenocarcinoma.
Discussion
To the best of our knowledge, this is the first to demonstrate the clinical efficacy of pyrotinib in a cervical cancer patient harboring HER2 G292R who progressed on radio-chemotherapy.
Currently, no HER2 or pan-HER inhibitors have been approved for HER2-mutant cervical cancers, owing to the fact that the clinical efficacy of HER2 or pan-HER inhibitors against HER2 mutations remains elusive in cervical cancer. HER2 G292R, which affects the extracellular domain of HER2, has been identified in non-small-cell lung cancer, breast cancer, and gallbladder carcinoma.Citation6,Citation7 HER2 G292R potentially leads to a gain of function of HER2 protein. In vitro studies have demonstrated that overexpression of HER2 G292R increase gallbladder cancer cell proliferation.Citation6 This mutation has not been reported in cervical cancers before.
Pyrotinib has been approved for HER2-positive breast cancer.Citation5 Previous studies have demonstrated the efficacy of pyrotinib against HER2-mutant solid tumors. A phase I basket trial completed in the United States has showed that patients with HER2-mutant lung cancer and other solid tumors obtained clinical benefit from pyrotinib treatment with a median progression-free survival (PFS) of 4.4 and 5.3 months, respectively.Citation8 A multicenter, open-label Phase II study has demonstrated that pyrotinib showed promising antitumor activity with an objective response rate of 30% and a PFS of 6.9 months in Chinese patients with HER2-mutant lung adenocarcinoma who progressed on platinum-based chemotherapy.Citation9 However, patients with cervical cancer or harboring HER2 G292R are not included in the above studies. Several activating HER2 mutations occurring in the extracellular domain of HER2 protein have been identified in solid tumors, including G265K, G292R, G309A/E, S310F, S310Y, and E405D.Citation4,Citation6,Citation10,Citation11 HER2 G309A/E, S310F, and E405D mutations have been shown to respond to afatinib or neratinib in vitro or in vivo.Citation4,Citation10 A prior study has demonstrated that 5.8% of cervical tumors carry HER2 extracellular domain mutations.Citation4 The most common subtype of HER2 mutations in cervical cancer is S310F/Y,Citation4 in contrast to the HER2 exon 20 insertions and V777/L755 missense mutations most commonly found in lung cancer and breast cancer, respectively. Cervical cancers carrying S310F or E405D are sensitive to afatinib or neratinib in vitro.Citation4 The SUMMIT basket trial has demonstrated the promising activity of neratinib in patients with breast, cervical, and biliary cancers carrying HER2 mutations.Citation12 The updated results from SUMMIT basket trial have demonstrated that 4 of 16 HER2-mutant cervical cancer patients derived objective response to neratinib with a median PFS of 7.0 months. All responders harbored HER2 S310F mutations, including one who achieved complete response to neratinib.Citation13 In this study, the HER2 G292R-positive patient derived complete remission of the primary cervical adenocarcinoma when treated with radio-chemotherapy. The patient subsequently derived complete remission of the metastatic tumors and remained in complete remission of the primary cervical tumor when treated with pyrotinib with a PFS of 25 months and counting. Both previous studies and ours suggest that HER2 extracellular domain mutations might be the promising therapeutic target in cervical cancer. Large cohorts and clinical trials are needed to further evaluate the efficacy of pan-HER inhibitor pyrotinib against HER2 G292R and other HER2 extracellular domain mutations in cervical cancer.
In summary, we revealed the first clinical evidence that a metastatic cervical adenocarcinoma patient carrying HER2 G292R mutation achieved CR with a PFS of 25 months and counting upon pyrotinib treatment. Our data shed light on the treatment options for previously treated advanced cervical adenocarcinoma patients harboring this or other activating HER2 mutations.
Ethics Statement
This study was approved by the Ethics Committee of Fujian Cancer Hospital. Patient provided informed consent to the study and permitted the use of tumor tissue. Written informed consent was obtained from the patient for publication of this case report.
Disclosure
The authors have no conflicts of interest to declare.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi:10.3322/caac.21590
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
- NCCN Clinical Practice Guidelines in Oncology. Cervical cancer Version 1.2021. Available from: www.nccn.org. Accessed August 31, 2021.
- Zammataro L, Lopez S, Bellone S, et al. Whole-exome sequencing of cervical carcinomas identifies activating ERBB2 and PIK3CA mutations as targets for combination therapy. Proc Natl Acad Sci U S A. 2019;116(45):22730–22736. doi:10.1073/pnas.1911385116
- Blair HA. Pyrotinib: first global approval. Drugs. 2018;78(16):1751–1755. doi:10.1007/s40265-018-0997-0
- Li M, Zhang Z, Li X, et al. Whole-exome and targeted gene sequencing of gallbladder carcinoma identifies recurrent mutations in the ErbB pathway. Nat Genet. 2014;46(8):872–876. doi:10.1038/ng.3030
- Chakravarty D, Gao J, Phillips SM, et al. OncoKB: a precision oncology knowledge base. JCO Precis Oncol. 2017;1:1–6. doi:10.1200/po.17.00011
- Li BT, Li T, Johnson ML, et al. Safety and efficacy of pyrotinib in patients with NSCLC and other advanced solid tumors with activating HER2 alterations: a Phase I basket trial. J Clin Oncol. 2020;38(15_suppl):3510. WCLC Abastrac #3510. doi:10.1200/JCO.2020.38.15_suppl.3510
- Zhou C, Li X, Wang Q, et al. Pyrotinib in HER2-mutant advanced lung adenocarcinoma after platinum-based chemotherapy: a multicenter, open-label, single-arm, Phase II Study. J Clin Oncol. 2020;38(24):2753–2761. doi:10.1200/jco.20.00297
- Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. doi:10.1158/2159-8290.cd-12-0349
- Greulich H, Kaplan B, Mertins P, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109(36):14476–14481. doi:10.1073/pnas.1203201109
- Hyman DM, Piha-Paul SA, Won H, et al. HER kinase inhibition in patients with HER2- and HER3-mutant cancers. Nature. 2018;554(7691):189–194. doi:10.1038/nature25475
- Oaknin A, Friedman CF, Roman LD, et al. Neratinib in patients with HER2-mutant, metastatic cervical cancer: findings from the Phase 2 SUMMIT basket trial. Gynecol Oncol. 2020;159(1):150–156. doi:10.1016/j.ygyno.2020.07.025
