Abstract
In 2012, prostate cancer will once again be the second-leading cause of cancer death of American males. Although initially treatable, prostate cancer can recur in a hormone refractory form that is not responsive to current available therapies. The mortality rate associated with hormone refractory prostate cancer is high, and there is an urgent need for new therapeutic agents to treat prostate cancer. A common feature of prostate cancer is the dependence on activated signal transducer and activator of transcription 3 (STAT3), a transcription factor, for survival. More important, inhibition of STAT3 has been shown to induce apoptosis in prostate cancer cells. In recent years, inhibitors of STAT3 have emerged as promising molecular candidates for targeted prostate cancer therapy. The aim of this review is to examine the role of STAT3 in prostate cancer and how inhibitors of STAT3 could advance the quest for treatment of the disease. Janus kinase 2 (JAK2)-targeted therapy appears very promising in the treatment of prostate cancer. It has been shown to decrease symptoms associated with myeloproliferative disorders and increase overall survival of patients compared with the best available therapy. In addition to improved outcome, many JAK2 inhibitors have been found to be tolerable with no adverse impact on quality of life. As such, JAK2 inhibitors may play an important role in the management of patients with prostate cancer. Current studies are evaluating the role of JAK2 inhibitors in solid tumors. Pending clinical trial results will determine the future direction of JAK2 inhibitors in the treatment of patients with prostate cancer.
Introduction
It is estimated that there will be 241,740 new prostate cancer cases in 2012, with a projected death toll of 28,170 within the same year.Citation1 Once again prostate cancer will be the second-leading cause of cancer death of American males. Current treatment options available for prostate cancer include (1) active surveillance, (2) surgery, (3) radiation therapy, (4) hormone therapy, (5) chemotherapy, and (6) immunotherapy.Citation2 The treatment given varies and it depends on age, overall health of individual, and the stage of disease. Prostate cancer, although initially treatable, can recur in an androgen-insensitive or hormone-refractory form that is not responsive to current therapies.Citation3 The mortality rate associated with recurrent prostate cancer is high; therefore, effective therapies to treat the disease, especially those adequate for recurrent cases, are in great demand.
Novel therapeutic agents designed to specifically target prostate cancer are needed. Targeted prostate cancer therapy using inhibitors of the signal transduction and activator of transcription 3 (STAT3) appears promising. A common feature of many prostate cancers is their dependence for survival on the activated form of STAT3. Importantly, inhibition of STAT3 has been shown to induce apoptosis in prostate cancer cells.Citation4–Citation6 The targeting of STAT3 could in practice serve as a suitable option for therapeutic intervention. This review will focus on STAT3, its role in prostate cancer, and how inhibitors of STAT3 could advance the quest for treatment of the disease.
STATs
Once activated, transcription factors are proteins that regulate the genome by either inducing or repressing gene expression. Transcription factors bind to specific DNA sequences in the genome upstream or near the promoter region of their gene of interest. STATs are now known to activate many genes involved in malignant progression and have recently emerged as ideal molecular targets for cancer therapy.Citation7–Citation9 STATs were originally discovered in their role as cytokine signaling proteins and comprise seven members: STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6.Citation10
The general structure of STATs includes a STAT dimerization domain at the NH2 terminus, a coiled-coil domain involved in protein–protein interactions, a central DNA binding domain, a Src homology 2 domain, and a COOH terminus encoding the transcription activation domain.Citation11,Citation12 STATs are activated in response to ligation of receptors by cytokines, hormones, and growth factors through phosphorylation of tyrosine and serine residues.Citation11,Citation12 For example, signaling by the interleukin 6 (IL-6) family generally induces phosphorylation of STAT3.Citation13,Citation14 Once phosphorylated, STATs undergo a conformational rearrangement; dimerization then occurs through interactions between phosphotyrosine and the Src homology 2 domain.Citation15 After activation, phosphorylated STATs dimers translocate to the nucleus and bind enhancer elements of target genes. In normal cells, the activation of STATs is tightly regulated and transient. However, constitutive activation of STATs has been associated with the malignant state. Constitutive activation of STAT3 in particular has been shown to be addictive: disrupting activation or expression or nuclear translocation leads to apoptosis of transformed but not benign cells.Citation5,Citation6,Citation16
Role of STAT3 in cancer
Originally known as acute-phase response factor, STAT3 was identified and cloned within the IL-6 pathway as a mediator of the acute-phase inflammatory response.Citation17,Citation18 However, it is now known that STAT3 is activated by an array of ligand-receptor interactions, including those mentioned earlier (cytokines, hormones, and growth factors), and also by activation of intracellular kinases.Citation11,Citation12 STAT3 can also be activated by growth factor receptors that possess intrinsic tyrosine-kinase activity. These include epidermal growth factor receptor, hepatocyte growth factor receptor (also known as c-Met), and the platelet-derived growth factor receptor.Citation19–Citation23 Non-receptor, cytoplasmic tyrosine kinases (ie, Abelson leukemia protein and Src-related kinases) also activate STAT3.Citation24–Citation26
In benign cells, STAT3 activation is initiated by the binding of a ligand to its receptor, which leads to dimerization of the cytoplasmic domain of the receptor and activation of associated Janus tyrosine kinases (Janus kinase 1 [JAK1], Janus kinase 2 [JAK2], or tyrosine kinase 2 [TYK2]) (). Activated upstream kinases in turn phosphorylate STAT3 on its conserved tyrosine residue (Tyr705), which allows for dimerization of STAT3. The STAT3 dimer then translocates to the nucleus where it regulates transcription. Dimerization of receptors with intrinsic tyrosine kinase activity can also directly phosphorylate STAT3.
Figure 1 Signal transducer and activator of transcription 3 (STAT3) activation: STAT3 activation is initiated by binding of the ligand, interleukin 6 (IL-6), to its receptor, which consists of glycoprotein 80 kDA and glycoprotein 130 kDA (gp130). Binding of IL-6 to the receptors leads to dimerization of the cytoplasmic domain of the gp130 peptide, with subsequent activation of associated Janus tyrosine kinases, notably Janus kinase 2 (JAK2). The activated JAKs in turn phosphorylate STAT3, which allows for dimerization of STAT3. The STAT3 dimer then translocates to the nucleus where it regulates transcription.
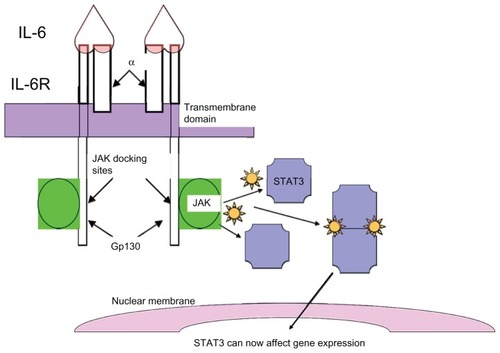
In malignant cells, the requirement for ligand binding is often lost. A number of human cancers have been reported to express constitutively activated STAT3Citation12,Citation27 – these include myeloma, lymphoma, head and neck squamous cell carcinoma, melanoma, and breast, ovarian, lung, pancreatic, and prostate cancer. Glycoprotein 130 kDA–mediated STAT3 activation, in particular, has been linked to many types of solid and hematological tumors.Citation28–Citation30 The glycoprotein 130 kDA receptor complex, part of the IL-6 signal transduction pathway, is well known for intracellular activation of JAK-TYKs as well as STATs. This mechanism of ligand-independent activation may be important in cancer.
STAT3 is essential for embryonic stem cell growth and renewal; disruption of STAT3 activity results in embryonic lethality in mice.Citation31,Citation32 STAT3-responsive elements have been identified in several genes influencing cell cycle progression, apoptosis, or metastasis. These genes include cyclin D1, B-cell lymphoma extra large, B-cell lymphoma 2, and matrix metalloproteinase 9.Citation33–Citation37 The upregulation of the expression of these genes in cancers is well known.Citation38–Citation43 STAT3 is also an important mediator of angiogenesis, as it has been shown to be required for vascular endothelial growth factor signaling.Citation44–Citation46 When persistently activated, STAT3 behaves as a proto-oncogeneCitation47–Citation50 and is believed to be required for the malignant phenotype. The mechanism of action of how STAT3 becomes persistently activated is unknown; although, in bladder cancer, the CDC91 L1 oncogene, activated by a chromosomal translocation, leads to persistent STAT3 activation. Citation51 In prostate cancer, abnormal activation of STAT3 is thought to be responsible for neoplastic progression of prostate cells.Citation52 Prostate cancer cells also expressing persistently activated STAT3 have been shown to become dependent on it for survival; disruption of expression of persistently activated STAT3 in prostate cancer cells results in apoptosis.Citation5,Citation6,Citation16 This makes STAT3 an excellent molecular candidate for prostate cancer therapy.
In recent years, STAT3 inhibitors have emerged as promising anticancer therapies.Citation53–Citation55 STAT3 signaling is advantageous because (1) constitutive activation of STAT3 is seen in many cancers; (2) STAT3 is associated with many genes involved in cell cycle progression, apoptosis, and metastasis; and (3) STAT3 is a single target. The interruption of STAT3 signaling can prompt the development of molecules effective in the treatment of a variety of tumors.
Different strategies have been established to inhibit STAT3 activity (). The most popular approach inhibits STAT3 by disrupting upstream tyrosine kinases (JAK1, JAK2, or TYK2) responsible for its activation. Other approaches such as oligonucleotides (antisense, decoy, g-quartet, and binding sequences), dominant-negative expression vectors, and siRNA methodologies target STAT3 directly.Citation6,Citation12,Citation56–Citation60 Oligonucleotides, in particular, are very attractive in theory, but a practical method to deliver oligonucleotides in a clinical setting has not been established. However, a possible approach to introduce oligonucleotides in a clinical setting is to use peptide-mediated transport, coupling a cell-penetrating peptide to a therapeutic payload (ie, peptide nucleic acid).Citation61,Citation62 Cell-penetrating peptides are advantageous because cell specificity in the sequence and organelle specificity, using nuclear localization signals, can be achieved. Less popular approaches target (1) physiological regulators of STAT3 (ie, suppressor of cytokine signaling [SOCS] proteins and protein inhibitors of activated STATs [PIAS]) or (2) STAT3 interacting nuclear proteins that help with STAT3 transcription regulation.Citation12 SOCS proteins and PIAS are negative regulators of STAT3 that block STAT3 activation.Citation63–Citation65 SOCS proteins bind to JAK1, JAK2, or TYK2 to inhibit STAT3 activity; PIAS interact directly with STAT3 and block its DNA-binding activity. However, these proteins are not totally specific for STAT3, as they have other functions in the cell. This makes them less attractive for therapeutic development.
Table 1 Pharmacological strategies to inhibit signal transducer and activator of transcription 3 (STAT3) signaling: different methods have been established including indirect, direct, or alternate inhibition of STAT3
Studies have shown that inhibition of STAT3 leads to cessation of tumor cell growth and apoptosis. Experiments conducted in the human myeloma cell line U266 resulted in two major findings: (1) the cell line was sensitive to AG490 (a JAK2 inhibitor that disrupts STAT3 activation; also a potent inhibitor of epidermal growth factor receptor, which can also interrupt STAT3 activity) and (2) the transfection of the cell line with a dominant-negative form of STAT3 (DN-STAT3), which blocks STAT3 function, induced apoptosis.Citation66 In another study, the transfection of DN-STAT3 in head and neck squamous cell carcinoma lines lead to the failure of cells to divide.Citation67 The use of DN-STAT3 also showed therapeutic ability in a melanoma model.Citation68 AG490 has also been shown to suppress growth in the classic cell lines of prostate cancer, DU-145 and PC3.Citation69 Another prostate cancer cell line, TSU-Pr1, was also sensitive to the effects of AG490 in the same study.
JAK2 inhibitors: a closer look
STAT3 signaling by cytokines is mediated through upstream tyrosine kinases JAK1, JAK2, or TYK2. Because of their downstream targets involved in apoptosis, proliferation, and differentiation, these tyrosine kinases are also potential therapeutic targets for cancer therapy. The development of JAK2 inhibitors, in particular, has recently been of great interest to researchers.Citation70–Citation74 A single point mutation within JAK2 was found to be linked to many myeloproliferative disorders.Citation75–Citation79 A substitution of valine by phenylalanine at amino acid 617 (JAK2V617F) was found in 95% of patients with polycythemia vera (PV), and 50% of patients with essential thrombocythemia (ET), or primary myelofibrosis.Citation55–Citation59 Point mutations or insertions/ deletions in JAK2 exon 12 have also been implicated in JAK2V617F-negative PV patients.Citation80–Citation83 The JAK2V617F mutation is located within JAK2’s autoregulatory JAK homology 2 pseudokinase domain, which, interestingly, lacks catalytic activity.Citation84 However, the JAK2V617F mutation causes JAK2 to be constitutively activated and results in increased activity and phosphorylation of downstream targets such as STATs.Citation79 JAK2 inhibitors appear promising in the treatment of hematological and solid tumor cancers that show aberrant activation of the JAK2-STAT signaling pathway.
Ruxolitinib (Incyte Corporation, Wilmington, DE), also known as INCB018424, is a potent inhibitor of JAK1 and JAK2.Citation85 It was among the first JAK2 inhibitors tested in myeloproliferative disorders. Ruxolitinib administered to JAK2V617F-positive mice reduced levels of circulating inflammatory cytokines and splenomegaly, which ultimately increased survival.Citation68,Citation86 Although the precise mode of action of ruxolitinib is not well understood, it is believed to competitively interact with the adenosine triphosphate (ATP) or substrate- binding site of JAK1 and JAK2.Citation71 Ruxolitinib showed promising results in phase III clinical trials from patients with myelofibrosis (primary, post-PV, or post-ET myelofibrosis); more than 35% of patients demonstrated reduction in spleen size and more than 50% showed reduction in myelofibrosis-related symptoms when ruxolitinib was taken orally twice daily, compared with the best available therapy (hydroxyurea or glucocorticoids) and placebo.Citation69 The US Food and Drug Administration approved ruxolitinib in November 2011 as the first drug treatment ever for myelofibrosis; ruxolitinib also was the first JAK2 inhibitor approved for therapy.Citation86 Clinical trials to study the effects of orally administered ruxolitinib in patients with androgen-independent, metastatic prostate cancer started in March 2008.Citation87 The study successfully advanced to phase II clinical trials. Patients were administered ruxolitinib 25 mg tablets twice daily with water over 21-day cycles for as long as the drug was tolerated. However, as of January 2012, the study was terminated after it was shown that fewer than two of the 22 patients showed a prostate-specific antigen response of 50.Citation87
AZD1480 is a potent inhibitor of JAK1 and JAK2. In preclinical studies, AZD1480 inhibited tumor growth in constitutively activated STAT3 solid tumor cell lines.Citation57 These cell lines, including DU-145 (a classic human prostate cancer cell line), had constitutive STAT3 action via IL-6 stimulation. AZD1480 also blocked constitutive STAT3 signaling and phosphorylation following suppression of tumor growth.Citation57 AZD1480 is now being studied in phase I/II clinical trials in patients with myelofibrosis and phase I clinical trials, alone or with docetaxel (Sanofi-Aventis, Paris, France), in patients with advanced solid malignancies.Citation88
Numerous other novel JAK2 inhibitors are still being studied in clinical trials (). TG101348 is another potent inhibitor of JAK2.Citation72 TG101348, an ATP-competitive inhibitor, resides in the ATP-binding pocket of JAK2 where it exemplifies its inhibitory effects.Citation72 TG101348 disrupts JAK2-induced phospho-STAT3 signaling in cultured and primary cells from patients with JAK2V617F-positive myeloproliferative disorders.Citation89,Citation90 TG101348 is now being studied in phase I/II clinical trials in patients with myelofibrosis. SB1518, CYT387, NS-018, CEP-701, and XL019 are also JAK2 inhibitors in phase I/II clinical trials for treatment in patients with myeloproliferative disorders, with promising future clinical trials extending to patients with solid malignancies.Citation70,Citation73,Citation91–Citation94
Table 2 Novel Janus kinase (JAK) inhibitors in development: numerous JAK1 and JAK2 inhibitors are being developed in clinical trials for treatment of myeloproliferative diseases and solid malignancies
Conclusion
Constitutively activated STAT3 has been implicated in a number of human cancers, including prostate cancer. As a result, STAT3 inhibitors have emerged as ideal molecular targets for cancer therapy. Targeted prostate cancer therapy using JAK2 inhibitors, in particular, appears very promising in the treatment of the disease. The study of JAK2 inhibitors in myeloproliferative disorders has provided a basis for the study of these novel molecules in solid tumors. JAK2 targeted therapy has been shown to decrease symptoms associated with myeloproliferative disorders and increase overall survival of patients compared with the best available therapy. In addition to improved outcome, many JAK2 inhibitors have been found to be tolerable with no adverse impact on quality of life. As such, JAK2 inhibitors may play an important role in the management of patients with prostate cancer. Current studies are evaluating the role of JAK2 inhibitors in solid tumors. Pending clinical trial results will determine the future direction of JAK2 inhibitors in the treatment of patients with prostate cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2012CA Cancer J Clin2012621102922237781
- National Cancer InstituteProstate cancer treatment (PDQ®)Bethesda, MDNational Cancer Institute2011 Available from: http://www.cancer.gov/cancertopics/pdq/treatment/prostate/Patient/page4Accessed Dec 16, 2011
- DenmeadeSRIsaacsJTProgrammed cell death (apoptosis) and cancer chemotherapyCancer Control19963430330910765221
- LouWNiZDyerKTweardyDJGaoACInterleukin-6 induces prostate cancer cell growth accompanied by activation of STAT3 signaling pathwayProstate200042323924210639195
- MoraLBBuettnerRSeigneJConstitutive activation of STAT3 in human prostate tumors and cell lines: direct inhibition of STAT3 signaling induces apoptosis of prostate cancer cellsCancer Res200262226659666612438264
- BartonBEKarrasJGMurphyTFBartonAHuangHFSignal transducer and activator of transcription 3 (STAT3) activation in prostate cancer: direct STAT3 inhibition induces apoptosis in prostate cancer linesMol Cancer Ther200431112014749471
- BowmanTGarciaRTurksonJJoveRSTATs in oncogenesisOncogene200019212474248810851046
- DarnellJEJrSTATs and gene regulationScience19972775332163016359287210
- BrombergJDarnellJEJrThe role of STATs in transcriptional control and their impact on cellular functionOncogene200019212468247310851045
- DarnellJEJrKerrIMStarkGRJak -STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteinsScience19942645164141514218197455
- DarnellJEJrTranscription factors as targets for cancer therapyNat Rev Cancer200221074074912360277
- YuHJoveRThe STATs of cancer: new molecular targets come of ageNat Rev Cancer2004429710514964307
- IhleJNSTATs and MAPKs: obligate or opportunistic partners in signalingBioessays199618295988851041
- AaronsonDSHorvathCMA road map for those who don’t know JAK-STATScience200229655731653165512040185
- ChenXVinkemeierUZhaoYJeruzalmiDDarnellJEJrKuriyanJCrystal structure of a tyrosine phosphorylated STAT-1 dimer bound to DNACell19989358278399630226
- BartonBEMurphyTFShuPHuangHFMeyenhoferMBartonANovel single-stranded oligonucleotides that inhibit signal transducer and activator of transcription 3 induce apoptosis in vitro and in vivo in prostate cancer cell linesMol Cancer Ther20043101183119115486184
- WegenkaUMBuschmannJLüttickenCHeinrichPCHornFAcute- phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational levelMol Cell Biol19931312762887678052
- AkiraSNishioYInoueMMolecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathwayCell199477163717512451
- SilvennoinenOSchindlerCSchlessingerJLevyDERas-independent growth factor signaling by transcription factor tyrosine phosphorylationScience19932615129173617398378775
- ZhongZWenZDarnellJEJrSTAT3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6Science1994264515595988140422
- Ruff-JamisonSZhongZWenZChenKDarnellJEJrCohenSEpidermal growth factor and lipopolysaccharide activate STAT3 transcription factor in mouse liverJ Biol Chem19942693521933219358071311
- HungWElliottBCo-operative effect of c-Src tyrosine kinase and STAT3 in activation of hepatocyte growth factor expression in mammary carcinoma cellsJ Biol Chem200127615123951240311278729
- ZhangYWWangLMJoveRVande WoudeGFRequirement of STAT3 signaling for HGF/SF-Met mediated tumorigenesisOncogene200221221722611803465
- ParsonsJTParsonsSJSrc family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathwaysCurr Opin Cell Biol1997921871929069259
- IrbyRBYeatmanTJRole of Src expression and activation in human cancerOncogene200019495636564211114744
- DanialNNRothmanPJAK-STAT signaling activated by Abl oncogenesOncogene200019212523253110851051
- BuettnerRMoraLBJoveRActivated STAT signaling in human tumors provides novel molecular targets for therapeutic interventionClin Cancer Res20028494595411948098
- Stuhlmann-LaeiszCLangSChalarisAForced dimerization of gp130 leads to constitutive STAT3 activation, cytokine-independent growth, and blockade of differentiation of embryonic stem cellsMol Biol Cell20061772986299516624864
- SriuranpongVParkJIAmornphimolthamPPatelVNelkinBDGutkindJSEpidermal growth factor receptor-independent constitutive activation of STAT3 in head and neck squamous cell carcinoma is mediated by the autocrine/paracrine stimulation of the interleukin 6/ gp130 cytokine systemCancer Res200363112948295612782602
- SelanderKSLiLWatsonLInhibition of gp130 signaling in breast cancer blocks constitutive activation of STAT3 and inhibits in vivo malignancyCancer Res200464196924693315466183
- NiwaHBurdonTChambersISmithASelf-renewal of pluripotent embryonic stem cells is mediated via activation of STAT3Genes Dev19981213204820609649508
- BoeufHHaussCGraeveFDBaranNKedingerCLeukemia inhibitory factor-dependent transcriptional activation in embryonic stem cellsJ Cell Bio19971386120712179298977
- FukadaTHibiMYamanakaYTwo signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosisImmunity1996554494608934572
- PuthierDBatailleRAmiotMIL-6 up-regulates Mcl-1 in human myeloma cells through JAK/STAT rather than ras/MAP kinase pathwayEur J Immunol199929123945395010602002
- KarniRJoveRLevitzkiAInhibition of pp60c-Src reduces Bcl-xL expression and reverses the transformed phenotype of cells overexpressing EGF and HER-2 receptorsOncogene199918334654466210467412
- IvanovVNBhoumikAKrasilnikovMCooperation between STAT3 and c-jun suppresses Fas transcriptionMol Cell20017351752811463377
- SinibaldiDWhartonWTurksonJBowmanTPledgerWJJoveRInduction of p21WAF1/CIP1 and cyclin D1 expression by the Src oncoprotein in mouse fibroblasts: role of activated STAT3 signalingOncogene200019485419542711114718
- KijimaTNiwaHSteinmanRASTAT3 activation abrogates growth factor dependence and contributes to head and neck squamous cell carcinoma tumor growth in vivoCell Growth Differ200213835536212193474
- LebedevaIRandoROjwangJCossumPSteinCABcl-xL in prostate cancer cells: effects of overexpression and down-regulation on chemosensitivityCancer Res200060216052606011085527
- LiXMaraniMMannucciROverexpression of Bcl-xL underlies the molecular basis for resistance to staurosporine-induced apoptosis in PC-3 cellsCancer Res20016141699170611245486
- YoshidaTHanadaTTokuhisaTActivation of STAT3 by the hepatitis C virus core protein leads to cellular transformationJ Exp Med2002196564165312208879
- YuQGengYSicinskiPSpecific protection against breast cancers by cyclin D1 ablationNature200141168411017102111429595
- MasudaMSuzuiMYasumatuRConstitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinomaCancer Res200262123351335512067972
- BartoliMPlattDLemtalsiTVEGF differentially activates STAT3 in microvascular endothelial cellsFASEB J200317111562156412824281
- YahataYShirakataYTokumaruSNuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formationJ Biol Chem200327841400264003112874294
- NiuGWrightKLHuangMConstitutive STAT3 activity up-regulates VEGF expression and tumor angiogenesisOncogene200221132000200811960372
- YuCLMeyerDJCampbellGSEnhanced DNA-binding activity of a STAT3-related protein in cells transformed by the Src oncoproteinScience1995269522081837541555
- GarciaRYuCLHudnallAConstitutive activation of STAT3 in fibroblasts transformed by diverse oncoproteins and in breast carcinoma cellsCell Growth Differ1997812126712769419415
- BrombergJFHorvathCMBesserDLathemWWDarnellJEJrSTAT3 activation is required for cellular transformation by v-srcMol Cell Biol1998185255325589566875
- TurksonJBowmanTGarciaRCaldenhovenEDe GrootRPJoveRSTAT3 activation by Src induces specific gene regulation and is required for cell transformationMol Cell Biol1998185254525529566874
- GuoZLinnJFWuGCDC91L1 (PIG-U) is a newly discovered oncogene in human bladder cancerNat Med200410437438115034568
- BartonBEMurphyTFAdemPWatsonRAIrwinRJHuangHFIL-6 signaling by STAT3 participates in the change from hyperplasia to neoplasia in NRP-152 and NRP-154 rat prostatic epithelial cellsBMC Cancer200111911710966
- FrankDASTAT signaling in cancer: insights into pathogenesis and treatment strategiesCancer Treat Res200311526729112613201
- Catlett-FalconeRDaltonWSJoveRSTAT proteins as novel targets for cancer therapy: signal transducer an activator of transcriptionCurr Opin Oncol199911649049610550013
- TurksonJJoveRSTAT proteins: novel molecular targets for cancer drug discoveryOncogene200019566613662611426647
- KonnikovaLKoteckiMKrugerMMCochranBHKnockdown of STAT3 expression by RNAi induces apoptosis in astrocytoma cellsBMC Cancer200332313678425
- LeongPLAndrewsGAJohnsonDETargeted inhibition of STAT3 with a decoy oligonucleotide abrogates head and neck cancer cell growthProc Natl Acad Sci U S A200310074138414312640143
- LewisHDWinterAMurphyTFTripathiSPandeyVNBartonBESTAT3 inhibition in prostate and pancreatic cancer lines by STAT3 binding sequence oligonucleotides: differential activity between 5′ and 3′ endsMol Cancer Ther2008761543155018566225
- SenMToscaPJZwayerCLack of toxicity of a STAT3 decoy oligonucleotideCancer Chemother Pharmacol200963698399518766340
- JingNZhuQYuanPLiYMaoLTweardyDJTargeting signal transducer and activator of transcription 3 with G-quartet oligonucleotides: a potential novel therapy for head and neck cancerMol Cancer Ther20065227928616505101
- Said HassaneFSalehAFAbesRGaitMJLebleuBCell penetrating peptides: overview and applications to the delivery of oligonucleotidesCell Mol Life Sci201067571572619898741
- LewisHDHusainADonnellyRJCreation of a novel peptide with enhanced nuclear localization in prostate and pancreatic cancer cell linesBMC Biotechnol2010107921029412
- StarrRHiltonDJNegative regulation of the JAK/STAT pathwayBioessays1999211475210070253
- NakaTFujimotoMKishimotoTNegative regulation of cytokine signaling: STAT-induced STAT inhibitorTrends Biochem Sci1999241039439810500304
- ShuaiKModulation of STAT signaling by STAT-interacting proteinsOncogene200019212638264410851063
- Catlett-FalconeRLandowskiTHOshiroMMConstitutive activation of STAT3 signaling confers resistance to apoptosis in human U266 myeloma cellsImmunity199910110511510023775
- GrandisJRDrenningSDChakrabortyARequirement of STAT3 but not STAT1 activation for epidermal growth factor receptor-mediated cell growth In vitroJ Clin Invest19981027138513929769331
- NiuGHellerRCatlett-FalconeRGene therapy with dominant-negative STAT3 suppresses growth of the murine melanoma B16 tumor in vivoCancer Res199959205059506310537273
- NiZLouWLemanESGaoACInhibition of constitutively activated STAT3 signaling pathway suppresses growth of prostate cancer cellsCancer Res20006051225122810728680
- Quintás-CardamaAKantarjianHCortesJVerstovsekSJanus kinase inhibitors for the treatment of myeloproliferative neoplasias and beyondNat Rev Drug Discov201110212714021283107
- WalzCCrossNCVan EttenRAReiterAComparison of mutated ABL1 and JAK2 as oncogenes and drug targets in myeloproliferative disordersLeukemia20082271320133418528425
- WernigGKharasMGOkabeREfficacy of TG101348, a selective JAK2 inhibitor, in treatment of a murine model of JAK2V617F-induced polycythemia veraCancer Cell200813431132018394554
- PardananiALashoTSmithGBurnsCJFantinoETefferiACYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patientsLeukemia20092381441144519295546
- HedvatMHuszarDHerrmannAThe JAK2 inhibitor AZD1480 potently blocks STAT3 signaling and oncogenesis in solid tumorsCancer Cell200916648749719962667
- JamesCUgoVLe CouédicJPA unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia veraNature200543470371144114815793561
- KralovicsRPassamontiFBuserASA gain-of-function mutation of JAK2 in myeloproliferative disordersN Engl J Med2005352171779179015858187
- LevineRLWadleighMCoolsJActivating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosisCancer Cell20057438739715837627
- BaxterEJScottLMCampbellPJAcquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disordersLancet200536594641054106115781101
- ZhaoRXingSLiZIdentification of an acquired JAK2 mutation in polycythemia veraJ Biol Chem200528024227882279215863514
- ScottLMTongWLevineRLJAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosisN Engl J Med2007356545946817267906
- PietraDLiSBrisciASomatic mutations of JAK2 exon 12 in patients with JAK2 (V617F)-negative myeloproliferative disordersBlood200811131686168917984312
- PardananiALashoTLFinkeCHansonCATefferiAPrevalence and clinicopathologic correlates of JAK2 exon 12 mutations in JAK2V617F-negative polycythemia veraLeukemia20072191960196317597810
- WangYLVandrisKJonesAJAK2 mutations are present in all cases of polycythemia veraLeukemia2008226128918079740
- SaharinenPTakaluomaKSilvennoinenORegulation of the JAK2 tyrosine kinase by its pseudokinase domainMol Cell Biol200020103387339510779328
- Quintás-CardamaAManshouriTEstrovZPreclinical characterization of atiprimod, a novel JAK2 and JAK3 inhibitorInvest New Drugs201129581882620372971
- US Food and Drug AdministrationJakafi (Ruxolitinib) labelSilver Spring, MDFDA2011 Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/202192lbl.pdfAccessed Jan 4, 2012
- ClinicalTrials.govStudy of ruxolitinib (INCB018424) administered orally to patients with androgen independent metastatic prostate cancerBethesda, MDNational Institutes of Health2012 Available from: http://clinicaltrials.gov/ct2/show/study/NCT00638378?sect=X3015&view=resultsAccessed February 13, 2012
- ClinicalTrials.govStudy to assess safety, tolerability and PK of AZD1480 alone or in comb with docetaxel in patients with solid tumoursBethesda, MDNational Institutes of Health2012 Available from: http://clinicaltrials.gov/ct2/show/NCT01112397?term=AZD1480&rank=2Accessed Feb 13, 2012
- PardananiAJAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trialsLeukemia2008221233017882282
- PardananiAHoodJLashoTTG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutationsLeukemia20072181658166817541402
- TynerJWBummTGDeiningerJCYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasmsBlood2010115255232524020385788
- HartSGohKCNovotny-DiermayrVSB1518, a novel macrocyclic pyrimidine-based JAK2 inhibitor for the treatment of myeloid and lymphoid malignanciesLeukemia201125111751175921691275
- NakayaYShideKNiwaTEfficacy of NS-018, a potent and selective JAK2/Src inhibitor, in primary cells and mouse models of myeloproliferative neoplasmsBlood Cancer J201117e29
- SantosFPKantarjianHMJainNPhase 2 study of CEP-701, an orally available JAK2 inhibitor, in patients with primary or postpolycythemia vera/essential thrombocythemia myelofibrosisBlood201011561131113620008298