Abstract
Aim:
Both of the diagnosis and treatment evaluation are time-consuming conditions in patients with pulmonary and pleural tuberculosis. The aim of this study was to establish the validity of tumor markers CA 125, CA 15-3, and CA 19-9 in the diagnosis of pulmonary and pleural TB and to verify the success of the treatment protocol.
Patients and methods:
The levels of tumor markers CA 125, CA 15-3, and CA 19-9 were measured before and after treatment in 67 TB patients, 54 of whom had pulmonary TB and 13 of whom had pleural TB. All values were compared with the results of a healthy control group of 44 subjects.
Results:
CA 125 and CA 15-3 levels were significantly high when compared with those of the healthy control group and there was a significant decrease in both tumor marker levels after treatment in patients with pulmonary TB (P < 0.001 and P < 0.004, respectively). However, the difference found in CA 19-9 levels before and after treatment in patients with pulmonary TB was not statistically significant (P < 0.08). When the CA 125, CA 15-3, and CA 19-9 values of the pulmonary TB group before treatment were compared with that of the healthy control group, the results were statistically significant in all parameters except CA 19-9 (P < 0.001, P < 0.001, and P < 0.09 for CA 125, CA 15-3, and CA 19-9, respectively). In the patients with pleural TB, CA 125, CA 15-3, and CA 19-9 values did not change significantly after treatment.
Conclusion:
The authors suggest that CA 125 and CA 15-3 tumor markers may be important for verification of the success of treatment protocol in pulmonary TB, as the differences found for these tumor markers between the pre- and the posttreatment periods are statistically significant.
Introduction
Pulmonary and pleural tuberculosis (TB) are both important public health issues today in Turkey and other parts of the world.Citation1–Citation3 Although the diagnosis of TB can be made easily, the standard isolation methods currently in use are quite slow – culture on conventional Löwenstein-Jensen medium requires 4–8 weeks. The spread of drug-resistant TB has emphasized the need for rapid diagnosis. There are many methods to assess the activity of pulmonary TB. The most widely used technique for diagnosing active TB is sputum smear microscopy.Citation4 However, in cases where a patient’s sputum smear is negative, a decision for treatment can be made based on typical clinical symptoms, specific findings on a chest roentgenogram, a tuberculin skin test, and sometimes typical findings on high-resolution computed tomography.Citation5 Recently, other methods such as measurement of serum neopterin and serum procalcitonin have been used in the diagnosis of TB.Citation5–Citation7 In pulmonary TB, efficacy of treatment is determined by an acid-resistant bacilli screening at the fifth month – but, that is a very long-time period for most patients. Some screenings performed during this period can verify the success of the treatment protocol (ie, can assess the response to the treatment). Pathological evaluation is preferred in the diagnosis of pleural TB.Citation8 Adenosine deaminase measurement in pleural fluid is another diagnostic method used.Citation9 There is also literature suggesting that certain tumor markers such as cancer antigen (CA) 125 are used both in diagnosis of TB and in evaluation of the efficacy of treatment.Citation10 Although studies with these markers are generally focused on TB peritonitis, some investigators have studied these markers in patients with pulmonary and pleural TB. CA 125 and CA 15-3 are the markers mainly studied. Very few studies of these markers have included CA 19-9.Citation11–Citation17 The aim of this study was to investigate the importance of measuring these tumor markers before and after treatment of pulmonary and pleural TB to assess the disease activity and to monitor response to therapy.
Material and methods
Sixty-seven patients with pulmonary (n = 54) and pleural (n = 13) TB were enrolled in this prospective study over approximately a 2-year period (March 2005 to April 2007). Local ethics committee approval was obtained for the study, and informed consent was obtained from all study participants. The TB patients did not have any disease other than TB. Serum samples were obtained from the patients with pulmonary or pleural TB and were analyzed by bacteriological, pathological, or radiological methods to measure CA 125, CA 15-3, and CA 19-9. This procedure was repeated after a 6-month anti-TB treatment (isoniazid, rifampicin, ethambutol, and pyrazinamide for the first 2 months; then isoniazid and rifampicin for the remaining 4 months). Because the study was performed in a military hospital, all patients were male (mean age, 25 ± 4.1 years). The authors recruited 44 healthy male subjects (mean age, 29 ± 6.7 years) as the control group. Samples were obtained only once from the control group.
In 35 of the patients with pulmonary TB, sputum smear and culture results were positive, whereas 19 of the patients with pulmonary TB had negative sputum smear but positive culture results. Diagnoses of pleural TB were made based on cytological analysis of pleural fluid, increased pleural fluid adenosine deaminase levels, or histological examination of pleural biopsy specimens in twelve patients. A pleurectomy was needed in only one patient for diagnosis and treatment. The 54 patients with pulmonary TB were grouped according to the degree of sputum smear positivity as 1,2,3 and 4 positivity. The scoring for degree of acid-resistant bacilli positivity can be summarized as follows: 1 if there is one to two bacilli in 100 areas, 2 if there is one to nine bacilli in 10 areas, 3 if there is one to nine bacilli in each area, and 4 if there is more than nine bacilli in each area. The results for the TB patients were compared with the results of the healthy control group, and the results obtained before and after treatment were compared.
Obtained serums were stored at −80C° and were analyzed simultaneously. CA 125, CA 15-3, and CA 19-9 levels were measured using the electrochemiluminescence immunoassay technique and an Analytics E170 (Elecsys module) immunoassay analyzer (Roche Diagnostics GmbH, Mannheim, Germany).
Statistical analyses were made using statistical software (SPSS programme 15.0; SPSS Inc, Chicago, IL). Descriptive statistics were shown as mean plus or minus standard deviation. For the statistical assessment of pre- and posttreatment results, paired sample t-test and Mann-Whitney U test were used. Correlations were determined by using Pearson and Spearmen tests. P-values < 0.05 were accepted as statistically significant.
Results
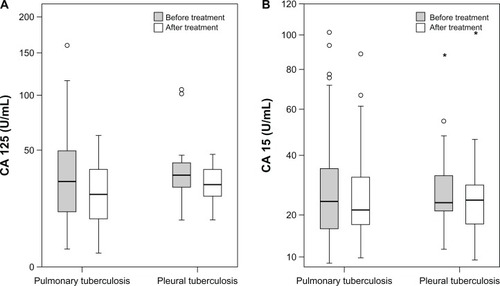
As mentioned earlier, the study involved 54 patients with pulmonary TB, 13 patients with pleural TB, and 44 healthy volunteers (control group) as study groups. Sputum smear results of the 54 patients with pulmonary TB (data not shown) were as follows: four positive acid-fast bacilli in five patients (9.2%), three positive in 16 patients (33.7%), two positive in seven patients (7.7%), and one positive in seven patients (7.7%); sputum smears (in three samples) failed to demonstrate acid-fast bacilli in 19 patients (28.4%), but culture results were positive. Pretreatment tumor markers of the patients with pulmonary (n = 54) and pleural TB (n = 13) were compared with the results of the healthy control group ( and ). A statistically significant difference was found in the pulmonary TB patients for CA 125 and CA 15-3 levels but not for CA 19-9 levels (). In the patients with pleural TB, the difference was not statistically significant when compared with the healthy control group ().
Table 1 The levels of tumor markers in patients with pulmonary tuberculosis before treatment and in the healthy control group
Table 2 The levels of tumor markers in patients with pleural tuberculosis before treatment and in the healthy control group
Mean CA 125, CA 15-3, and CA 19-9 values before and after treatment in the pulmonar y TB patients () were as follows: CA 125, 40.94 ± 34.54 U/mL (pretreatment) versus 26.33 ± 15.46 U/mL (posttreatment) (P < 0.001) (); CA 15-3, 30.97 ± 21.49 U/mL (pretreatment) versus 26.83 ± 15.47 U/mL (posttreatment) (P < 0.004) (); and CA 19-9, 10.40 ± 13.89 U/mL (pretreatment) versus 11.16 ± 15.66 U/mL (posttreatment) (P = 0.08). In the patients with pleural TB there was no statistically significant difference found between the values (): CA 125, 41.82 ± 29.23 U/mL (pretreatment) versus 28.96 ± 10.61 U/mL (posttreatment) (P = 0.06); CA 15-3, 31.92 ± 21.13 U/mL (pretreatment) versus 29.38 ± 23.61 U/mL (posttreatment) (P = 0.20); and CA 19-9, 5.29 ± 2.98 U/mL (pretreatment) versus 5.16 ± 2.84 U/mL (posttreatment) (P = 0.18).
Table 3 The pre- and posttreatment levels of tumor markers in patients with pulmonary tuberculosis
Table 4 The pre- and posttreatment levels of tumor markers in patients with pleural tuberculosis
Higher serum CA 125 levels were obtained from the patients with a higher degree of sputum smear positivity (r = 0.341, P = 0.012). This significant correlation was not found for the other tumor markers (CA 15-3: r = 0.032, P = 0.817; CA 19-9: r = −0087, P = 0.532).
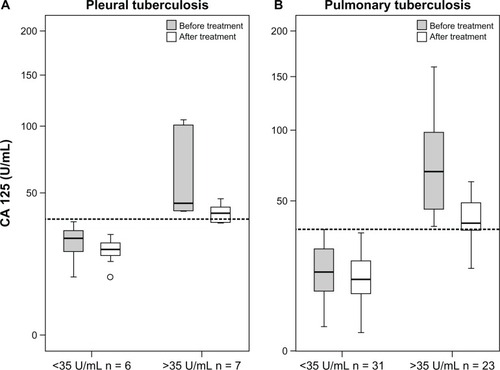
Another interesting finding for CA 125 was that if the CA 125 value was higher than the reference value (35 U/mL) at the moment of diagnosis, a significant decrease was observed after treatment in both pulmonary and pleural TB patients (P < 0.001). However, if the CA 125 value was not high before the treatment, this reduction was not significant ().
Discussion
CA 125 is a protein found on the surface of many ovarian cancer cells. It also can be found in other cancers and on a limited scale in normal tissue.Citation18 Most often, the CA 125 test is used in diagnosing ovarian cancer and to check how well treatment for ovarian cancer is working or whether the ovarian cancer has relapsed. In some nonmalignant diseases (chronic renal failure, some autoimmune diseases, granulomatous liver diseases, pancreatitis, and pulmonary TB) elevated values can be detected.Citation14
Some recent studies have reported increased serum CA 125 values found in peritoneal and pleural TB patients.Citation19,Citation20 Ozsahin et alCitation10 showed that CA 125 measurement may be useful in the diagnosis of TB and in differentiation of active and inactive disease.Citation10 Some articles have claimed that CA 125 can be used to follow up the efficacy of treatment. The measurement was performed before treatment and after 4, 6, and 36 months of treatment. When the cutoff value was taken as 31 U/L, sensitivity and specificity were high (97% and 100%, respectively). The decrease was found to be statistically significant during this period.Citation21
The present authors determined that normal CA 125 levels increase in pulmonary and pleural TB. This increase was positively correlated with the degree of smear positivity in pulmonary TB. In other words, a patient with a degree of sputum smear positivity of 4 had a higher serum CA 125 level than a patient with a negative smear. A significant decrease to the normal level was obtained with a 6-month anti-TB treatment (). This decrease in CA 125 levels was obvious if the preliminary value was higher than the cutoff value of 35 U/mL (). Therefore, the present authors claim that CA 125 can be used in the monitoring of treatment response in pulmonary TB patients. Fortún et alCitation22 reported that CA 125 values increase in patients with pulmonary TB and decrease to normal values during treatment. According to reported articles, the CA 125 level evaluated in patients with a negative sputum acid-fast bacillus stain.Citation22 Huang et alCitation23 suggested that CA 125 serum levels – in combination with clinical responses, chest radiography, and sputum examinations – can offer improved monitoring of therapeutic responses in anti-TB treatment.Citation23
CA 15-3 is a normal product of breast cells; it is produced by a gene that is often overexpressed in cancerous breast tumors, leading to an increased production of CA 15-3.Citation15 However, CA 15-3 may also be elevated in some nonmalignant diseases such as chronic hepatitis, cirrhosis, sarcoidosis, TB, and systemic lupus erythematosus.Citation15,Citation16,Citation24,Citation25 A value greater than 25 U/mL is considered high.
Colomer et alCitation15 determined an increase in CA 15-3 values in approximately 10% of patients with active TB.Citation15 However, there are not enough data that demonstrate the role of CA 15-3 in evaluating the response to anti-TB treatment. The present study is, to the best of the authors’ knowledge, the first study to establish the relationship between CA 15-3 and treatment response.
CA 19-9 is a tumor-associated marker. It is synthesized by normal human pancreatic and bile duct cells, as well as by gastric, colonic, endometrial, salivary, and bronchial epithelia.Citation12,Citation13,Citation17 Immunohistochemically, CA 19-9 is expressed in mucous cells of the bronchial gland and the surface of the bronchiolar epithelium cells in benign pulmonary disease.Citation12 Usually it can be detected in malignant diseases but high levels are rarely found in benign liver and gallbladder diseases.Citation17
Takayama et alCitation12 measured serum CA 19-9 levels of 156 patients with benign pulmonary diseases. In 13 patients with healed pulmonary TB, the percentage of the patients with positive serum CA 19-9 was 61.5%. In the present study there was no significant correlation found between the disease activity and the response to treatment.
As already mentioned, there were 13 patients with pleural TB included in the present study. No significant increase was found in any parameter for these patients. If the patients were evaluated individually, both CA 125 and CA 15-3 levels were found to have decreased at the end of the treatment. However, it is possible that the number of patients in the study was not large enough for a statistical evaluation.
Although the routine use of tumor markers in all patients with TB cannot be approved, tumor markers can be useful in verification of the success of treatment protocol (ie, tumor markers can be useful in assessment of the response to treatment).
Conclusion
The CA 125 and CA 15-3 tumor markers correlate well with the disease activity in pulmonary TB. These two tumor markers may be used to assess the response to treatment. The authors conclude that benign pulmonary diseases such as pulmonary TB affect serum tumor markers. In this study the authors suggest that determination of CA 125 and CA 15-3 levels in patients with pulmonary TB may be useful both in the diagnosis of illness and as a measure to provide verification of the efficacy of treatment protocol (ie, assess the response to treatment).
Disclosure
The authors report no conflicts of interest in this work.
References
- TanrikuluACAcemogluHPalanciYDagliCETuberculosis in Turkey: high altitude and other socio-economic risk factorsPublic Health2008122661361918294666
- SevimTAtaçGGüngörGTreatment outcome of relapse and defaulter pulmonary tuberculosis patientsInt J Tuberc Lung Dis20026432032511936741
- AktogğuSYorganciogluACirakKKöseTDereliSMClinical spectrum of pulmonary and pleural tuberculosis: a report of 5,480 casesEur Respir J1996910203120358902463
- KilicaslanZKiyanEKucukCRisk of active tuberculosis in adult household contacts of smear-positive pulmonary tuberculosis casesInt J Tuberc Lung Dis2009131939819105885
- OrsFDenizOBozlarUHigh-resolution CT findings in patients with pulmonary tuberculosis: correlation with the degree of smear positivityJ Thorac Imaging200722215415917527119
- GülerMHüddamDUnsalEThe role of serum neopterin level in the evaluation of activation and response to treatment in the patients with pulmonary tuberculosisTuberk Toraks2006544330335 Turkish.17203418
- BalbayÖATürkerGÇalisşirHCŞengülAAs a diagnostic markers of active pulmonary tuberculosis procalcitonin placeSolunum200011299303 Turkish.
- ChikumiHShimizuEDiagnostic significance of pleural effusion and pleural biopsyNihon Naika Gakkai Zasshi2000895874883 Japanese.10853472
- ChenMLYuWCLamCWAuKMKongFYChanAYDiagnostic value of pleural fluid adenosine deaminase activity in tuberculous pleurisyClin Chim Acta20043411–210110714967164
- OzsahinSLTurgutBNurNDoganOTErselcanTBerkSValidity of the CA125 level in the differential diagnosis of pulmonary tuberculosisJpn J Infect Dis2008611686918219138
- WaniAMAkhtarMCA-125: a marker for diagnosis and follow-up of pleuroperitoneal and lymph node tuberculosisAnn Saudi Med200828214214318398279
- TakayamaSKataokaNUsuiYCA 19-9 in patients with benign pulmonary diseasesNihon Kyobu Shikkan Gakkai Zasshi1990281013261331 Japanese.2273661
- KomiyaTMatsushimaTKimuraMAdachiMA case of endobronchial tuberculosis with high serum CA19-9 and SLX levelKekkaku19946910615619 Japanese.7799572
- YilmazAEceFBayramgürlerBAkkayaEBaranRThe value of Ca 125 in the evaluation of tuberculosis activityRespir Med200195866666911530955
- ColomerRRuibalAGenolláJCirculating CA 15-13 levels in the postsurgical follow-up of breast cancer patients and in non-malignant diseasesBreast Cancer Res Treat19891321231332730960
- UstünHBorazanABilgiçliNYılmazADiagnostic value of tumoural markers in pleural effusionsInt J Clin Pract2004581222514994966
- IshiuraYFujimuraMMinamiSIncreased CA19-9 level in serum and bronchoalveolar lavage fluid from a patient with pulmonary tuberculosisNihon Kyobu Shikkan Gakkai Zasshi1996344477481 Japanese.8691672
- SimsekHSavasMCKadayifciATatarGElevated serum CA 125 concentration in patients with tuberculous peritonitis: a case-control studyAm J Gastroenterol1997927117411769219793
- MasMRCömertBSağlamkayaUCA-125: a new marker for diagnosis and follow-up of patients with tuberculous peritonitisDig Liver Dis200032759559711142557
- UzunköyANazlıgülYPeritoneal tuberculosis: reviewTurkiye Klinikleri J Med Sci200626404408
- YılmazAEceFBayramgürlerBAkkayaEBaranRThe value of CA 125 in the evaluation of tuberculosis activityRespir Med200195866666911530955
- FortúnJMartín-DávilaPMéndezRCA-125: a useful marker to distinguish pulmonary tuberculosis from other pulmonary infectionsOpen Respir Med J2009312312719966922
- HuangWCTsengCWChangKMHsuJYChenJHShenGHUsefulness of tumor marker CA-125 serum levels for the follow-up of therapeutic responses in tuberculosis patients with and without serositisJpn J Infect Dis201164536737221937816
- GhayumiSMMehrabiSDoroudchiMGhaderiADiagnostic value of tumor markers for differentiating malignant and benign pleural effusions of Iranian patientsPathol Oncol Res200511423624116388321
- Schöniger-HekeleMMüllerCThe combined elevation of tumor markers CA 19-9 and CA 125 in liver disease patients is highly specific for severe liver fibrosisDig Dis Sci200651233834516534678