Abstract
EML4-ALK rearrangement is common in lung adenocarcinoma. The ALK inhibitors remarkably inhibit lung adenocarcinoma and reveal long-term beneficial effects in several patients. Advanced genetic testing technology reveals that EML4-ALK rearrangement has been observed in patients with lung squamous cell carcinoma. In the present study, we report a case of a 53-year-old patient with EML4-ALK rearranged in lung squamous carcinoma; PET/CT scan revealed brain and multiple bone metastases. First-line ALK-TKI combined with local stereotactic body radiation therapy indicated progression-free survival of 9 months. After progressive disease, treatment was switched to lorlatinib, with little efficacy and a total overall survival of 11 months. The emergence of drug resistance revealed that the genetic test result was EML4-ALK fusion (V3a/b variants), indicating a poor prognosis. In this study, we analyzed the treatment efficacy of ALK inhibitors and provided a research basis for the treatment of EML4-ALK rearranged in lung squamous cell carcinoma patients.
Introduction
The fusion between echinoderm microtubule-associated protein-like 4 (EML4) and anaplastic lymphoma kinase (ALK) has recently been identified in a subset of non-small-cell lung cancers (NSCLC). EML4-ALK rearrangement rate in NSCLC patients is approximately 3–7%Citation1 and is majorly observed in lung adenocarcinoma. Moreover, the application of ALK-tyrosine kinase inhibitor (TKI)-targeted drug conversion therapy reveals a chance of long-term survival in patients with advanced EML4-ALK-mutated NSCLC that cannot be operated; however, some patients cannot be cured.Citation2 Furthermore, the detection rate of EML4-ALK rearrangement mutations in patients with lung squamous carcinoma is approximately 1% with the clinical application of next-generation sequencing (NGS). Thomas Huang has reported a case of lung squamous cell carcinoma patient with a heavy smoking history who benefited from ALK inhibitors, wherein particularly brain metastases achieved complete response (CR).Citation3 The present study reports a case of EML4-ALK rearranged lung squamous cell carcinoma who was treated with second- and third-generation ALK-TKI combined with stereotactic body radiation therapy (SBRT) revealing remarkable clinical benefit.
Case Report
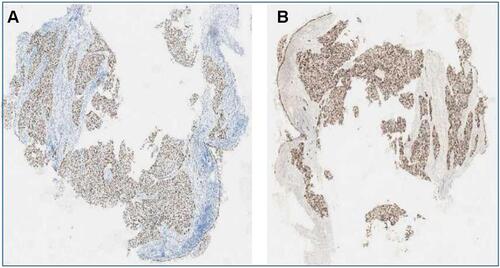
A 53-year-old Chinese male patient presented with 10 years smoking history (5 cigarettes per day) and had quit smoking for 10 years. In April 2020, the patient presented persistent pain in the right thigh and buttocks. A whole-body positron emission tomography (PET/CT) scan indicated the left lower lobe lesion, with multiple scattered nodules in both lungs. Hilar and mediastinum on both sides revealed numerous enlarged lymph nodes and increased fluorodeoxyglucose metabolism. Multiple osteolytic destruction occurred in the fifth rib axillary on the right, sixth thoracic vertebra, fifth lumbar vertebra, and the right transverse process and the right flank of the vertebral roots, sacral 1 and 2 vertebrae. The brain magnetic resonance imaging revealed enhanced nodules in the right hippocampus. The biopsy of the lower trachea was taken for pathological analyses and demonstrated squamous cell carcinoma. Immunohistochemistry (IHC) revealed that CK7, CK5/6, P63, and P40 were positive (), whereas TTF-1 and NapsinA were negative. Real-time polymerase chain reaction results indicated EML4-ALK/KIF5B fusion mutation (mutation Ct value 17.38).
Figure 1 Pulmonary tumor squamous cell histology analysis. (A) P40 immuno- histochemistry stain, 20×; (B) P63 immunohistochemistry stain, 20× magnification demonstrating squamous differentiation.
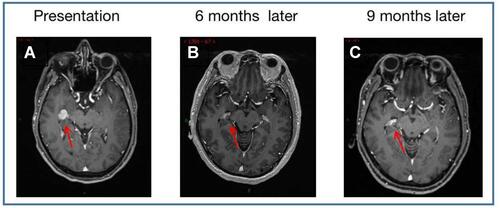
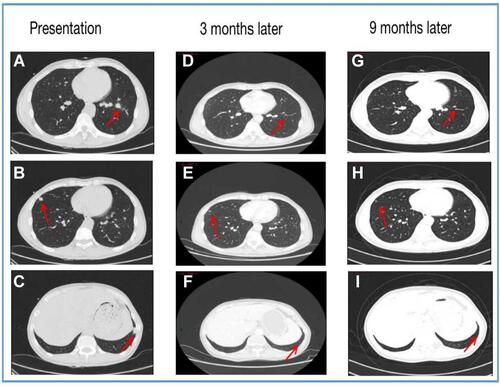
The patient was administered alectinib orally from June 2020; meanwhile, he received palliative SBRT local radiotherapy 30Gy/6f for lumbar spine, sacral, and surrounding soft tissue metastases; 50Gy/10f for brain metastases; and 36Gy/6f for right fifth rib metastases. The efficacy was assessed via part response (PR) through CT scan. In March 2021, imaging evaluation revealed CR in the intracranial metastases and both lung lesions ( and ); however, the mediastinal and cervical lymph nodes were larger than before, and new metastases appeared in the first lumbar vertebra. The disease was evaluated as progressive disease (PD), and PFS was obtained after receiving alectinib treatment for 9 months. In April 2021 post-progression, the next-generation sequencing (NGS) was performed on the formalin-fixed paraffin-embedded (FFPE) sample analysis with a YuanSu™ panel. NGS genetic testing reported an EML4-ALK fusion (V3a/b variants). Thereafter, the patient was subjected to the third-generation ALK inhibitor lorlatinib. In May 2021, CT scan indicated mediastinal lymph node continued progression, with an OS of 11 months. During the treatment, patients experienced adverse reactions such as edema and muscle soreness; however, no adverse reactions were greater than grade 3.
Discussion
The ALK fusion events account for approximately 3–7% genetic alterations in NSCLC patients. EML4 is the most common ALK fusion partner, accounting for 90–95% of ALK rearrangements. EML4-ALK is divided into multiple subtypes; among these, V1 (E13; A20) and V3 (E6; A20) account for the highest proportion (both at ~32%), whereas other EML4-ALK fusion subtypes are relatively rare (<10%).Citation4 In addition to EML4, variation in the rare ALK fusion partners such as KIF5B, TFG, KLC1, SOCS5, HIP1, TPR, and BIRC6 may be related to tumor progression and treatment.Citation5 Moreover, differences were observed in the resistance mutations produced by various fusion subtypes or different treatment schemes.Citation6 Alex reported that alectinib revealed promising efficacy with a median PFS as high as 34.8 months when it was used as first-line therapy in patients with advanced ALK-positive NSCLC.Citation7 In 2018, the National Comprehensive Cancer Network (NCCN) recommended alectinib as a first-line treatment for ALK-positive NSCLC patients.Citation8 Furthermore, lorlatinib, a third-generation ALK inhibitor, is a novel, reversible, and potent small-molecule ATP inhibitor of ALK and ROS1. It exhibited remarkable inhibitory effect on all known drug-resistant mutations of ALK and revealed high disease control rates in patients who previously underwent ALK inhibitor treatment, regardless of whether they received chemotherapy.Citation9
In the present case study, the patient was diagnosed to suffer from advanced lung squamous cell cancer with EML4-ALK/KIF5B fusion mutation, which was rare in patients with lung squamous cell cancer. Only few case reports of ALK-TKI-targeted therapy are available in patients with advanced lung squamous cell carcinoma,Citation10–Citation16 with a median PFS of 7.1 months. In this study, the patient received first-line oral alectinib treatment combined with SBRT specific for bone and cranial metastases and achieved a PFS of 9 months. Zeng Min reported that the application of EGFR-TKI combined with SBRT in treating oligometastatic advanced NSCLC markedly prolonged PFS and OS.Citation17 This may explain the prolonged PFS in this patient; however, the change in treatment to lorlatinib after emergence of drug resistance was almost ineffective, and the disease progressed further.
NSCLC patients with EML4-ALK rearrangements exhibited differential sensitivity and duration of targeted drugs. Presently, more than 17 EML4-ALK fusion variants have been identified,Citation18 the most common being 1, 2, and 3a/b, which account for 90% of the total fusion variants.Citation19 Based on the study of the molecular morphology of EML4-ALK, the focus of the current research has shifted to the correlation between EML4-ALK anomaly and response and whether specific fusion abnormalities will further affect the cancer spread, prognosis, and efficacy of ALK-targeted therapy.Citation20 Previous studies demonstrated that V3a/b variants were less sensitive to tyrosine kinase inhibitors compared to V1 and V2 variants;Citation21 moreover, patients with EML4-ALK V3a/b and V5a variants revealed significantly shorter PFS than those with V1 and V2 variants.Citation22 The detection of EML4-ALK V3 variants in this patient during subsequent dynamic genetic testing may be associated with a poor prognosis. At present, the role of EML4-ALK variants has not been fully elucidated. In the future, further research on the differences of EML4-ALK variants will help to develop new NSCLC treatment strategies for ALK mutation targeted therapy and improve the prognosis and survival of NSCLC treatment.
In summary, first-line ALK inhibitors are used in combination with SBRT to treat lung squamous carcinoma patient harboring EML4-ALK rearrangement, which has a longer PFS than only ALK-TKI. The EML4-ALK V3 variant was associated with a poor prognosis, despite undergoing treatment with the third-generation ALK-TKI lorlatinib.
Ethical Approval
Institutional approval was not required to publish the case details.
Patient Informed Consent
Written informed consent was obtained from the patient for the publication of his case details and images.
Acknowledgment
We are grateful to the patient and her family. And this work was funded by Sichuan Medical Association (S18043).
Disclosure
All authors disclose no conflicts of interest.
References
- Friboulet L, Li N, Katayama R, et al. The ALK inhibitor ceritinib overcomes crizotinib resistance in non-small cell lung cancer. Cancer Discov. 2014;4(6):662–673. doi:10.1158/2159-8290.CD-13-0846
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–1703. doi:10.1056/NEJMoa1006448
- Huang T, Engelmann BJ, Morgan RM, et al. EML4–ALK rearrangement in squamous cell carcinoma shows significant response to anti-ALK inhibitor drugs crizotinib and alectinib. Cancer Chemother Pharmacol. 2018;81(5):965–968. doi:10.1007/s00280-018-3571-2
- Lin JJ, Zhu VW, Yoda S, et al. Impact of EML4-ALK variant on resistance mechanisms and clinical outcomes in ALK-positive lung cancer. J Clin Oncol. 2018;36(12):1199–1206. doi:10.1200/JCO.2017.76.2294
- Song P, Zhang L, Shang CC, et al. Current status for anaplastic lymphoma kinase in non-small cell lung cancer. Zhongguo Fei Ai Za Zhi. 2018;2021(9):703–711. doi:10.3779/j.issn.1009-3419.2018.09.10
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016;6(10):1118–1133. doi:10.1158/2159-8290.CD-16-0596
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus crizotinib in untreated ALK-positive non-small cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi:10.1056/NEJMoa1704795
- Ettinger DS, Aisner DL, Wood DE, et al. NCCN guidelines insights: non- small cell lung cancer, version 5. 2018. J Natl Compr Canc Netw. 2018;16(7):807–821. doi:10.6004/jnccn.2018.0062
- Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015;28(1):70–81. doi:10.1016/j.ccell.2015.05.010
- Mikes RE, Jordan F, Hutarew G, et al. First line crizotinib in anaplastic lymphoma kinase (ALK) rearranged squamous cell lung cancer. Lung Cancer. 2015;90(3):614–616. doi:10.1016/j.lungcan.2015.10.013
- Srivastava N, Van der Laan PA, Kelly CP, et al. Esophagitis: a novel adverse event of crizotinib in a patient with ALK-positive non-small-cell lung cancer. J Thorac Oncol. 2013;8(3):e23–4. doi:10.1097/JTO.0b013e31827e2451
- Tamiya A, Shimizu S, Atagi S. A case of squamous cell carcinoma harboring an EML4-ALK rearrangement that was unsuccessfully treated with the ALK inhibitor alectinib. J Thorac Oncol. 2015;10(8):e74. doi:10.1097/JTO.0000000000000575
- Vergne F, Quere G, Andrieu-Key S, et al. ALK-rearranged squamous cell lung carcinoma responding to crizotinib: a missing link in the field of non-small cell lung cancer? Lung Cancer. 2016;91:67–69. doi:10.1016/j.lungcan.2015.11.010
- Wang Q, He Y, Yang X, et al. Extraordinary response to crizotinib in a woman with squamous cell lung cancer after two courses of failed chemotherapy. BMC Pulm Med. 2014;14:83. doi:10.1186/1471-2466-14-83
- Wang W, Song Z, Zhang Y. Response to crizotinib in a squamous cell lung carcinoma patient harbouring echinoderm microtubule-associated protein-like 4-anaplastic lymphoma translocation: a case report. Thorac Cancer. 2016;7(3):355–357. doi:10.1111/1759-7714.12298
- Zhang Q, Wang J, Zhang S. ALK-rearranged squamous cell lung cancer: a case report. Int J Clin Exp Pathol. 2015;8(2):2195–2198.
- Wang XX, Zeng M. First-line tyrosine kinase inhibitor with or without aggressive upfront local radiation therapy in patients with EGFRm oligometastatic non-small cell lung cancer: interim results of a randomized Phase III, open-label clinical trial (SINDAS) (NCT02893332). J Clin Oncol. 2020;38(15_suppl):9508. doi:10.1200/JCO.2020.38.15_suppl.9508
- Sarah SR, Yeoh S, Jackson G, et al. EML4-ALK variants: biological and molecular properties, and the implications for patients. Cancers (Basel). 2017;9(9):118. doi:10.3390/cancers9090118
- Soda M, Isobe K, Inoue A, et al. A prospective PCR-based screening for the EML4-ALK oncogene in non-small cell lung cancer. Clin Cancer Res. 2012;18(20):5682–5689. doi:10.1158/1078-0432.CCR-11-2947
- Christopoulos P, Endris V, Bozorgmehr F, et al. EML4-ALK fusion variant V3 is a high-risk feature conferring accelerated metastatic spread, early treatment failure and worse overall survival in ALK+ non-small cell lung cancer. Int J Cancer. 2018;142(12):2589–2598. doi:10.1002/ijc.31275
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung cancers. Sci Transl Med. 2012;4(120):120ra17. doi:10.1126/scitranslmed.3003316
- Liu XY, Liang HG, Wang MZ, et al. Therapeutic strategies for circumventing ALK inhibitors resistance in NSCLC. Zhonghua Zhong Liu Fang Zhi Za Zhi. 2018;25(4):298–302.