Abstract
β-adrenergic signaling modulates key signaling pathways that are important for tumor-promoting processes, and numerous mechanisms of action have been elucidated. Preclinical studies have demonstrated that β-adrenergic antagonists, or β-blockers, can block multiple fundamental biologic processes underlying the progression and metastasis of tumors, including the inhibition of cell proliferation, migration, invasion, resistance to programmed cell death, and tumor angiogenesis and metastasis. Human pharmacoepidemiologic studies suggest that β-blockers have a role in inhibiting cancer progression and metastasis in combination with standard therapies. Furthermore, a number of prospective studies have demonstrated that β-blockers are effective at halting infantile hemangioma growth. These findings shed light on the novel perspective of using β-blockers as a class of potential antitumor agents in clinical oncology.
Introduction
β-blockers are a class of drugs used for various indications, particularly for the management of cardiac arrhythmia, cardioprotection after myocardial infarction, hypertension, migraines, and tremors. The therapeutic activity of β-blockers is attributed to the blockade of β1-adrenergic receptors (ARs), which are predominantly expressed in cardiac tissue. β2-AR is expressed in the bronchiolar smooth muscle of the lung and other tissues and resembles β1-AR in its molecular and pharmacological properties. Accordingly, β-blockers can be classified by cardioselectivity and intrinsic sympathomimetic activity. Cardioselective β-blockers preferentially inhibit β1 receptors. Non-cardioselective β-blockers inhibit both β1- and β2-AR sites ().Citation1 Propranolol was the first clinically useful β-AR blocker and is nonselective for the β1- and β2-ARs. Invented by Sir James W Black, propranolol revolutionized the medical management of angina pectoris, and this drug is considered to be one of the most important contributions to clinical medicine and pharmacology of the 20th century.Citation2,Citation3
Table 1 Classes of β-blockers
The β-ARs, a family of G-protein-coupled receptors that are activated by β-adrenergic agonists, can initiate a series of signaling cascades, thereby leading to multiple, cell-specific responses. There is evidence suggesting that β-adrenergic signaling plays a role in basic developmental processes (eg, embryogenesis and morphogenesis), including the control of cell proliferation, differentiation and migration.Citation4–Citation6 Furthermore, β-adrenergic signaling has been found to regulate multiple biological processes that contribute to the initiation and progression of cancer. Based on this connection, emerging evidence suggests that β-blockers, either in vitro or in vivo, significantly reduce the proliferation, angiogenesis and metastasis of the most common human malignancies, including adenocarcinoma of the breast,Citation7,Citation8 lung,Citation9 pancreas,Citation10–Citation12 prostate,Citation13 colon,Citation14 and stomach,Citation15 as well as in ovarian cancer.Citation16 This finding has led to the hypothesis that commonly prescribed β-blockers may favorably impact cancer progression and metastasis in patients. In fact, the recent encouraging results from studies using β-blockersCitation4–Citation6 as a class of antitumor agents have been discovered as a result of decades of meticulous groundwork, followed by a few key observations in patients being treated with β-blockers in clinical trials. In this review, we will discuss the function of β-blockers, their antitumor activity in animal models and cell lines, and their potential as a novel tumor therapy.
Laboratory science
β-adrenergic signaling modulates multiple cellular processes
The neurotransmitters epinephrine and norepinephrine are the physiological agonists for β-ARs. These two neurotransmitters are catecholamines that are not only released from the adrenal medulla as a response to psychological and physical stress, but they also regulate cell and organ responses to the sympathetic branch of the autonomic nervous system. The synthesis and release of epinephrine and norepinephrine are regulated by nicotinic acetylcholine receptors (nAChRs).Citation17 Ligation of β-ARs by epinephrine or norepinephrine triggers a G-protein-coupled signaling cascade that stimulates cyclic adenosine monophosphate (cAMP) synthesis. This second messenger, cAMP, regulates many cellular functions through its effectors, such as cAMP-dependent protein kinase (PKA) and exchange proteins directly activated by cAMP (EPAC).Citation18,Citation19 PKA regulates a wide variety of cellular processes ranging from general metabolism and growth to cell-specific processes, such as differentiation, morphology, motility, secretion, neurotransmission, and gene transcription.Citation20 EPAC signaling accounts for many cAMP-induced effects on cell morphology, motility, and secretion dynamics.Citation21,Citation22
Blockade of β-ARs reduce tumor progression and metastasis
The possible causes and mechanisms of tumorigenesis have provoked much debate and controversy. However, out of this tumult has emerged the widely accepted principle that both the process of carcinogenesis and the resulting tumors are extremely complex. Preclinical studies have demonstrated that β-adrenergic signaling can regulate multiple fundamental biologic processes underlying the progression and metastasis of tumors, including promotion of inflammation,Citation23–Citation25 angiogenesis,Citation26,Citation27 migration,Citation28 invasion,Citation29 and resistance to programmed cell death.Citation30,Citation31 Some evidence suggests that the stimulation of β-adrenergic signaling can also inhibit DNA damage repairCitation32,Citation33 and cellular immune response,Citation34,Citation35 and promote surgery-induced metastasis.Citation16,Citation36,Citation37 Because β-adrenergic signaling can modulate multiple biologic processes and pathways underlying tumor progression and metastasis, β-blockers may be highly desirable for therapeutic intervention. Although the antitumor mechanisms of β-blockers are described separately below, there are numerous interactions among them, which indirectly reflect the complexity of tumor pathogenesis.
Angiogenesis
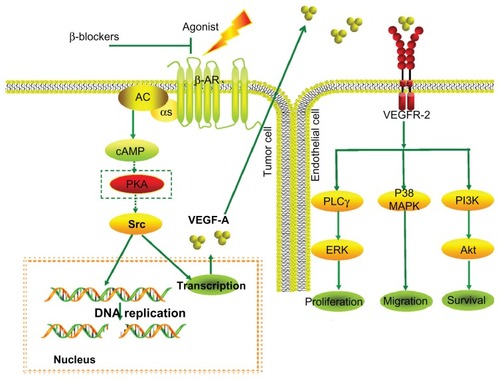
Angiogenesis is critical for tumor growth and progression. The neovessels of tumors are mainly formed from preexisting vessels via the proliferation and migration of endothelial cells together with the contribution of endothelial progenitor cells and endothelial stem cells.Citation38,Citation39 It is now well established that the overexpression of vascular endothelial growth factor (VEGF), a key proangiogenic protein, is associated with progression in several tumor types, including prostate cancer,Citation40 breast cancer,Citation41 hepatocellular carcinoma,Citation42 and infantile hemangioma (IH).Citation43 The β-ARs are important for mediating the production of this key proangiogenic cytokine. Exposure to a chronic stressor promoted in vivo angiogenesis and the production of VEGF. This effect was eliminated by silencing tumor cell β-AR expression, implicating tumor cell β-AR expression and signaling as important facilitators of stress-induced tumor angiogenesis in vivo.Citation27
In vitro studies using tumor cell lines suggest that catecholamines can promote tumor progression via a β-AR-driven, proangiogenic pathway. The stimulation of VEGF expression by β-adrenergic signaling is proportional to β-AR expression and is dose-dependent.Citation44 Conversely, β-blockers such as propranolol lead to the reduced expression of VEGF and thus to inhibition of angiogenesis (). Furthermore, the impairment of signal transduction at the β2-AR level in vascular endothelial cells can directly result in impairment to angiogenesis in vivo and tubulogenesis in vitro.Citation45,Citation46 It has been suggested that the benefits observed during IH treatment are also primarily due to the inhibition of VEGF production by propranolol.Citation47,Citation48 This suggestion has recently been confirmed by Chim et al,Citation49 who demonstrated that propranolol exerts its suppressive effects on hemangiomas through the hypoxia inducible factor (HIF)-1α–VEGF-A angiogenesis axis, the effects of which are mediated through the PI3/Akt and p38/MAPK pathways.
Figure 1 β-blockers abolish induction of VEGF expression by β-adrenergic agonists, leading to inhibition of angiogenesis.
Abbreviations: ARs, adrenergic receptors; cAMP, cyclic AMP; ERK, extracellular signal-regulated kinase; PI3K, phosphatidylinositol-3-kinase; PKA, cAMP-dependent protein kinase; PLCγ, phospholipase C-γ; MAPK, mitogen-activated protein kinase; VEGF, vascular endothelial growth factor.
Cell proliferation
Controlling cellular proliferation in tumors may be an effective treatment strategy. The stimulation of β-AR by lifestyle and environmental factors, as well as a preexisting risk for neoplasm, can activate downstream effector molecules and encourage cancer growth by promoting cell proliferation.Citation50,Citation51 The first evidence for a regulatory role of β-adrenergic signaling in cancer cells was provided by Schuller and Cole.Citation52 These researchers demonstrated a significant increase in the proliferation of lung adenocarcinoma cells in response to the β-AR agonist isoproterenol, with propranolol inhibiting the proliferation response. After this report, a number of studies have demonstrated that the epinephrine and norepinephrine neurotransmitters can induce cell proliferation in different cancer types.Citation9,Citation24,Citation53 These effects are mediated primarily through β2-AR activation in the cAMP-PKA signaling pathway in tumor cells.Citation54 However, there is evidence suggesting that epinephrine can directly stimulate esophageal cancer cell proliferation via the β1/β2-AR/ERK/COX-2 pathway and the β1-AR-dependent upregulation of cyclins and cyclin-dependent kinases.Citation55 Interestingly, inhibition of cell proliferation has also been described by agonists in breast cancer cells.Citation56 Moreover, the study by Carie and Sebti showed in vitro and in vivo a clear reduction of cell proliferation and tumor growth by a β-adrenergic agonist in breast and other cancers.Citation57
It is well known that nicotine can stimulate tumor growth and angiogenesis; however, nicotine itself does not cause neoplastic transformation.Citation17 Several studies have shown that nicotine-derived nitrosamine (4)-methylnitrosamino-1-(3-pyridl)-1-butanone (NNK) is a high-affinity agonist for both β-ARs and nAChRs.Citation58,Citation59 NNK can induce the development of pulmonary adenocarcinoma (PAC) in vivo, thereby indicating a direct and causative association between smoking and the incidence of PAC.Citation60 Subsequent investigations revealed that NNK stimulated the proliferation of PAC cells via a cAMP-dependent signaling cascade that includes the activation of the transcription factor cAMP response-element binding protein (CREB), the PKA-dependent epidermal growth factor receptor (EGFR) pathway, and the β-AR-mediated release of arachidonic acid.Citation58,Citation61,Citation62 Moreover, endogenous physiological epinephrine significantly increased NNK-induced cell proliferation. In contrast to these observed agonistic effects, propranolol completely abrogated NNK-induced proliferation.Citation63,Citation64
Apoptosis
Apoptosis is a form of cell death with features that are distinct from those of necrosis. It has become increasingly clear that the apoptotic cell death process is a relatively ubiquitous phenomenon that is observed in numerous cancer tissues and cell lines. There is evidence suggesting that β-AR may trigger multiple signaling pathways that could contribute to the induction of apoptosis, including the down-regulation of antiapoptotic proteins/genes and the activation of the caspase cascade.Citation51,Citation65,Citation66 Because resistance to apoptosis has been implicated in cancer pathogenesis, a number of studies have sought to analyze the effects of β-adrenergic signaling on apoptosis during tumor progression. In a study by Sastry et al,Citation67 the authors demonstrated that epinephrine reduces the sensitivity to apoptosis of both prostate cancer and breast cancer cells via β-AR/PKA signaling, which triggers BAD phosphorylation at S112, an effect that was completely reversed using a β2-AR-specific antagonist. Analogous to these findings, other studies determined that the inhibition of β-AR signaling using propranolol or a β2-AR antagonist in combination with gemcitabine induces apoptosis in pancreatic cancer cells.Citation68,Citation69 Furthermore, it was demonstrated that β-adrenergic inhibition by propranolol can enhance the effect of radiotherapy on gastric cancer cells in vitro through the induction of apoptosis via NFκB downregulation.Citation70
Resistance to anoikis is a hallmark of malignant transformation. Anoikis provides tumor cells with increased survival times in the absence of matrix attachment and facilitation migration, reattachment, and colonization of secondary sites.Citation71 Analysis of cellular models and an orthotopic mouse model of human ovarian cancer demonstrated that catecholamines can protect ovarian cancer cells from anoikis and that these effects are mediated by focal adhesion kinase (FAK) phosphorylation through the β2-AR-dependent activation of Src. Furthermore, all of these effects were blocked by propranolol or the β2-AR-specific antagonist butoxamine.Citation72 Additional studies using human clinical tumors showed that both depression and tumor norepinephrine content were associated with increased FAK activation and that increased FAK activation was associated with substantially accelerated disease progression.Citation72
Inflammation
Researchers estimate that inflammation may contribute to the development of up to 15% of all cancers.Citation73 It has been demonstrated that pro-inflammatory cytokines might promote tumorigenesis by inducing DNA damage or inhibiting DNA repair through the generation of reactive oxygen species.Citation35 Pro-inflammatory cytokines can also lead to the inactivation of tumor suppressor genes, the promotion of autocrine or paracrine growth, the survival of tumor cells, the stimulation of angiogenesis, or the subversion of the immune response.Citation35,Citation73 Among the pro-inflammatory cytokines displaying the striking effects was interleukin 6 (IL-6). IL-6 has been shown to participate in the epithelial–mesenchymal transition of human breast cancer cells.Citation74 IL-6 has also been shown to be secreted by ovarian cancer cells and to facilitate tumor cell proliferation, migration, and chemotherapy resistance.Citation75 In addition, IL-6 is a potent angiogenic cytokine in vivo.Citation76,Citation77
Elevated levels of IL-6 are frequently detected in the serum of breast cancer and ovarian cancer patients and are associated with poor prognosis and increased tumor burden. More importantly, both physical and psychological stressors can provoke transient, increased levels of pro-inflammatory cytokines. β-AR-induced IL-6 production has been observed in a variety of normal cell typesCitation78 and tumor cell types,Citation79 even in the absence of a pro-inflammatory stimulus.Citation80 There is evidence suggesting that chronic stress hormones affect IL-6 expression in breast and ovarian cancer through β-adrenergic signaling and contribute to angiogenesis in these tumors. Conversely, β-blockers such as propranolol have been shown to block many of the deleterious effects of stress.Citation81,Citation82 Another example involves chronic stress linked to IL-8, which is highly expressed in the majority of human cancers and has also been identified as a driver of tumor progression in various cancer types.Citation83,Citation84 It has been demonstrated that epinephrine and norepinephrine can enhance IL-8 expression through β2-AR and thereby mediate the effects of stress on the growth and metastasis of ovarian cancer; notably, these effects are all blocked by propranolol.Citation23
Immune response
Evidence from cancer patients clearly indicates that the immune system extensively interacts with developing primary tumors, metastasizing cells, and established metastases, and that the immune system can recognize and kill many malignant cells.Citation85 β-adrenergic signaling plays an important role in the regulation of tumor-directed immune responses. Both tumor-bearing animals and cancer patients have disrupted endocrine and immunological cycles, with greater disruption observed in cases where the tumor is advanced or fast-growing.Citation35,Citation86 Emerging evidence suggests that stress hormone catecholamines have specific effects on the immune systems of cancer patients. These effects include reducing lymphocyte proliferation,Citation87,Citation88 decreasing natural killer (NK) cell cytotoxicity,Citation35,Citation86 and reducing T-cell response to mitogen stimulation.Citation89 Catecholamines can also activate oncogenic viruses and alter antibody production, cytokine production profiles, and cell trafficking.Citation34 Most importantly, catecholamine can increase the prometastatic effects of the tumor immune response. Many tumors release catecholamines or recruit tumor-associated macrophages to do so, presumably as an immune-escape mechanism or to promote tumor vascularization.Citation7,Citation90
Migration and invasion
Activating the migratory ability of tumor cells is a prerequisite for tumor cell invasion and metastasis. There is evidence to suggest that tumor cell migration can be activated by signal substances from the neuroendocrine system. In vivo cancer models have shown that norepinephrine exerts both chemokinetic and chemoattractive effects on colon,Citation91 prostate,Citation92 and breast cancer cells.Citation93 By contrast, the norepinephrine-induced stimulation of cancer cell migration can be inhibited by propranolol or γ-aminobutyric acid (GABA)-mediated reduction of cAMP-dependent signaling.Citation94
Tumor cell invasion is a key step in the pathogenesis of metastasis. Matrix metalloproteinases (MMP) play a critical role in cell invasion by degrading components of the extracellular matrix. Indirect evidence suggests that catecholamines could potentially enhance tumor cell invasiveness because norepinephrine has been shown to affect tumor cell motility in vivo and because circulating catecholamines have been associated with the in vivo expression of MMPs that facilitate invasion.Citation91,Citation94–Citation97 Sood et alCitation29 established that physiologically relevant concentrations of norepinephrine and epinephrine can significantly enhance the capacity of ovarian tumor cells to invade the extracellular matrix via the β-adrenergic upregulation of MMP-2 and MMP-9. Additional findings by the same research group reported that the effects of norepinephrine and epinephrine are dependent on the activation of signal transducer and activator of transcription-3 (STAT-3) and proceeding through the β1/β2-AR and PKA.Citation98 Similarly, Yang et alCitation99 and Guo et alCitation100 determined that exposing nasopharyngeal carcinoma tumor cells and pancreatic cancer cells to norepinephrine resulted in increased production of the MMPs responsible for invasion responses; notably, these effects were completely blocked by propranolol.
Metastasis
Metastasis is the most common cause of morbidity and mortality in solid cancer patients. Psychosocial and physical stressors have been ascribed with playing a role in the incidence and metastasis of cancer.Citation97 Experimental stressors have been found to increase metastasis in various animal tumor models. There is evidence to suggest that social stress in mice increases the metastasis rates of breast cancer xenografts,Citation7 and that stress induced by passive restraint as well as treatment with epinephrine had similar effects on ovarian cancer metastasis.Citation29 Recent studies clearly indicate that physiological levels of catecholamines, or the release of these compounds after surgery, result in increased breastCitation36 and ovarianCitation16 carcinoma lymph node and lung metastases. Previously, the immune system had been attributed with functioning as a mediator between malignant tumors and the neuroendocrine system, and, as a consequence of this mediating role, immune suppression was hypothesized to enhance tumor establishment. Indeed, it has been demonstrated that the cytotoxicity of NK cells is strongly impaired by catecholamines, which might be supportive for the effects of metastasis formation.Citation35,Citation86 It has also been shown that chronic stress hormones can regulate breast cancer progression and metastasis by recruiting or modifying the activity of tumor-associated macrophages and the associated intratumoral expression of pro-metastatic genes, including VEGF, MMP-9, COX-2, transforming growth factor (TGF)-β and serum arginase (ARG)-1, as well as progression-inhibitory genes such as interferon (IFN)-β.Citation7
In addition to the role of the immune system at mediating stress effects on tumor growth and metastasis, there are now many reports indicating that chronic stress hormones can act directly by stimulating the pro-metastatic capacities of the malignant tissue and its microenvironment. These effects were mediated primarily through the activation of the tumor cell cAMP/PKA signaling pathway by β2-AR.Citation27,Citation99,Citation101,Citation102 Conversely, treatment with propranolol significantly reduces these effects. Interestingly, using the BALB/c nude mice model, Palm et alCitation13 observed that β-AR activation by norepinephrine had no effect on the growth of primary prostate cancer; however, there was an increase in the rate and magnitude of distant metastasis. In the study by Sloan et al,Citation7 stress-induced neuroendocrine activation actually had a negligible impact on primary tumor growth; however, this activation induced a 30-fold increase in metastasis to distant tissues. Similarly, pharmacological activation of β-adrenergic signaling by isoproterenol induced the same effects (ie, an increase in the number and mass of distant metastases by nearly 22-fold without substantially impacting primary tumor growth).Citation7 Perhaps most importantly, pretreating the animals with β-blockers synergistically blocked the effects of behavioral stress and/or β-adrenergic signaling activation on tumor metastasis. However, β-blockers have little effect on primary tumor growth.Citation7,Citation13 These findings lead to a conceptual change concerning the therapeutic role for β-blockers: β-blockers may protect against cancer progression and metastasis in already established cancers rather than preventing primary cancer occurrence or its cure.Citation103 These findings also suggest that clinically testing the use of β-blockers as an adjuvant therapy for the chemoprevention of metastasis development in cancer patients should be performed, especially with regard to the fact that the diagnosis of cancer itself and the according clinical treatment causes stress (eg, surgical stress).
Epidemiological and clinical studies
Do β-blockers influence cancer incidence?
As previously stated, multiple lines of evidence indicate that an elevated level of catecholamine stress neurotransmitters may be an etiological factor in various types of cancer. This hypothesis is immediately and, to some degree, directly testable by the epidemiological analysis of the medical records from people who have taken anti-catecholamine drugs such as β-blockers. A number of retrospective population studies suggest that β-blockers may have a protective role in reducing the incidence of all cancer types.Citation104–Citation106 In addition, the hypothesis of a protective effect of β-blockers against specific types of cancer is supported by studies of prostate cancerCitation107,Citation108 and colorectal cancer.Citation109 In contrast, several epidemiologic studies have identified a positive association between β-blockers and the risk of cancer.Citation110,Citation111 In a recent study by Jansen et al,Citation111 information on β-blocker use and potential confounders was collected for colorectal cancer cases and controls. The study concluded that there was no association between colorectal cancer and the use of β-blockers or any subclass of β-blockers after adjusting for confounding factors. However, the study indicated a positive association between long-term β-blocker use and the risk of stage IV colorectal cancer.
Other studies have found no relationship between β-blocker use and the risk of cancer.Citation109,Citation112 Data from studies on melanoma did not reveal any impact of β-blocker use on melanoma incidence.Citation113,Citation114 In addition, several epidemiologic studies examining the effect of β-blocker intake on breast cancer incidence have consistently found no significant link.Citation115,Citation116 Hence, the relationship between the use of β-blockers and cancer remains controversial. Recently, a meta-analysis using only randomized trials to minimize the effect of confounding factors was performed by Bangalore et al.Citation117 In this study, the authors identified 70 randomized controlled trials (148 comparator groups) with 324,168 participants. Reassuringly, their results suggest no evidence of even a 5%–10% relative risk increase in cancer or cancer-related deaths for any individual class of antihypertensive drugs (eg, β-blockers) studied.
Do β-blockers influence cancer progression?
β-blockers reduce melanoma progression
Two retrospective studies, by De Giorgi et alCitation113 and Lemeshow et al,Citation114 examined the association between the exposure of melanoma patients to β-blocker medication and survival. In the study by De Giorgi et al,Citation113 30 patients received β-blockers and were matched with 91 patients who were not treated. After adjusting for age and Breslow thickness, they concluded that β-blocker treatment was inversely associated with recurrence and that there was a significant reduction in the risk of relapse for each year of β-blocker use. The study by Lemeshow et alCitation114 used the national tumor registry in Denmark and a publicly available pharmacy database of 4179 patients diagnosed with melanoma with a median follow-up of 4.9 years. The authors compared melanoma patients receiving β-blockers (including metoprolol, propranolol, and atenolol) either within 90 days of diagnosis or more than 90 days prior to diagnosis, who were matched with patients not receiving a β-blocker. There was a significant reduction in melanoma-related death and all-cause mortality for the β-blocker users. The improved overall survival of melanoma patients receiving β-blockers following their diagnosis suggests that these compounds might prevent metastatic disease progression.
β-blockers reduce breast cancer metastasis, recurrence, and mortality
A retrospective study by Powe et alCitation118 reported on a study of 466 women with breast cancer who were treated for hypertension, with or without β-blocker medication. Women receiving β-blockers demonstrated a remarkable reduction in distant metastases and tumor recurrence and a 71% reduction in cancer-specific mortality. This was the first report in humans to suggest the protective effect of β-blockers in the treatment of breast cancer. In a subsequent study by Ganz et al,Citation119 the authors used observational data from 1779 women from the Kaiser Permanente Northern California Cancer Registry to examine the association between β-blockers and breast cancer recurrence, breast cancer-specific mortality, and overall mortality. Compared to women not exposed to β-blockers, women taking β-blockers displayed a nonsignificant 14% reduction in the risk of breast cancer recurrence, a nonsignificant 24% reduction in the risk of breast cancer-specific mortality, and no reduction in all-cause mortality.
Population studies by Barron et alCitation115 and Melhem-Bertrandt et alCitation116 also suggest that β-blockers may provide therapeutic leverage in the context of breast cancer. In the study by Barron et al,Citation115 the linked National Cancer Registry in Ireland and prescription dispensing data were used to identify women diagnosed with stages I to IV invasive breast cancer. The authors compared women taking either propranolol or atenolol during the year before their breast cancer diagnosis, and these women were matched (1:2) with women not taking β-blockers for age, socioeconomic factors, marital status, smoking status, tumor grade or size, chemotherapy, and use of long-term prophylactic medications (eg, statins or aspirin). The authors determined that propranolol users were significantly less likely to present with a T4, node-positive (N2/N3) or metastatic disease (M1) compared with the matched non-β-blocker users. Furthermore, a longer duration of propranolol use was associated with fewer T4 tumors, suggesting the possibility of a dose-dependent relationship. However, there was no significant difference in T4 or N2/N3/M1 tumor incidence and breast cancer-specific mortality between atenolol users and matched nonusers. In the study by Melhem-Bertrandt et al,Citation116 the authors retrospectively reviewed 1414 patients with breast cancer who received neoadjuvant chemotherapy. Medication usage in this study was obtained through patient self-reporting, as recorded in the medical records, and then extracted into the clinical research database. The most commonly prescribed β-blockers were metoprolol (42%) and atenolol (37%). Melhem-Bertrandt et alCitation116 compared the patients with and without β-blocker exposure for pathologic complete response (pCR), relapse-free survival (RFS), and overall survival. They found that pCR rates were not associated with β-blocker usage. However, β-blocker usage was associated with a significantly better RFS. Furthermore, β-blocker usage was associated with improved RFS among patients with triple-negative breast cancer. The clinical results from these reports strongly suggest that the effect of β-blockers on the treatment of breast cancer is dependent on protecting against cancer progression and metastasis on already established cancers rather than on the prevention of primary cancer occurrence or its cure.
β-blocker treatment of IH
IH, which is the most common infancy tumor, is a benign vascular neoplasm resulting from the abnormal proliferation of endothelial cells and angiogenesis. In 2008, Léauté-Labrèze et alCitation47 described their serendipitous observation of the anti-proliferative effect of propranolol on severe IHs. The authors described the rapid onset of the effect of propranolol treatment as an IH color change from red to purple within 24 hours, and they also observed a softening of the lesions. After this report, a number of studies further demonstrated that β-blockers other than propranolol (eg, timolol, acebutolol, and atenolol) were effective at halting hemangioma growth with few adverse side effects.Citation120–Citation124 The rapid action of β-blockers was especially dramatic in cases involving dyspnea, hemodynamic compromise, or palpebral occlusion. Another remarkable aspect of β-blocker treatment was that not only was the IH growth stabilized but improvement continued until complete involution was achieved, leading to a considerable shortening of the natural course of IH.Citation120 This medication may be particularly useful in clinical practice to hasten involution and as a replacement for early surgery or to lower the age at which timely surgery can be performed to maximize excision with minimal scarring.
A randomized, double-blind, placebo-controlled, parallel-group trial was conducted at a single institution in Australia by Hogeling et alCitation125 between June 2009 and December 2010. Forty children between the ages of 9 weeks and 5 years with cutaneous IH were included in the study. The study medication was dispensed at a ratio of 1:1 (placebo vs propranolol). Outcome measures included the blinded volume estimation, IH color (redness or blueness), and tumor elevation. The study concluded that propranolol users were more likely to show a significant reduction in both tumor volume and redness/elevation. Recently, another blinded cohort study by Pope et al,Citation126 explored the efficacy and safety of nadolol, a nonselective β-blocker with no intrinsic sympathomimetic activity, in patients with IH. A total of 19 patients were included in this study. Ten patients were recruited for the nadolol group, and 9 patients were recruited for the propranolol groups. The authors established that patients who were treated with nadolol had a more favorable response and fewer parental reports of minor adverse events than patients who were treated with propranolol.
Conclusion and future directions
The findings summarized above demonstrate that β-blockers have multiple biologic functions for inhibiting tumor progression and metastasis. The results from these preclinical and pharmacoepidemiologic studies support the need for further trials in clinical oncology. Because host factors (eg, adiposity and weight gain, physical activity, and alcohol and tobacco use),Citation115 confounding with other pharmacologic exposures (eg, angiotensin-converting enzyme inhibitors and aspirin)Citation115,Citation119 and comorbid medical conditions (eg, system inflammation or infectious disease)Citation127 can influence survival and recurrence after cancer treatment, further observational studies are not likely to definitively establish the clinical utility of β-blockers in cancer. Therefore, randomized controlled trials may provide the only way to overcome the selection and ascertainment bias.Citation50 Additionally, despite the apparent widespread use of β-blockers, the current data are limited, and many studies are unable to fully explore the dose–response relationship and the effects of β-blocker exposure on subgroups of patients due to limited sample sizes. Larger multicenter trials may provide more detailed information regarding the use of β-blockers in subsets of patients with various tumor types and provide the opportunity to definitively assess the protective effects of β-blockers on a clinical tumor population. It has also been suggested that nonselective blockers might be preferred over selective β1-blockers because immunocytes predominantly express β2-AR over β1-AR, and both have been implicated in tumor progression.Citation44,Citation55,Citation128 In addition, it was reported that the current selective β1-blockers in use are not entirely β1-specific. Indeed, all of them partially inhibit β2-AR.Citation129 It is therefore possible that even limited β2-adrenergic inhibition by β1-blockers might be sufficient to inhibit tumor progression.Citation130 Thus, nonselective blockers such as propranolol and nadolol should be proposed for consideration in future randomized controlled trials.
Authors’ contributions
YJ and SYC drafted the manuscript. KL, XMX, and SZ revised the manuscript. All of the authors have read and approved the final manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (Grants 81071903 and 81072069) (to Li); the Key Clinical Discipline of the Ministry of Health (Grant 201043941) (to Li); and the Mingdao Project of Fudan University (to Ji and Chen). No institution was involved in data interpretation, in writing the article, or in the decision to submit the paper for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
- HelfandMPetersonKChristensenVDanaTThakurtaSDrug Class Reviews: Beta Adrenergic Blockers: Final Report Update 4 [Internet]Drug Class ReviewsPortlandOregon Health and Science University2009
- FoodyJMFarrellMHKrumholzHMBeta-blocker therapy in heart failure: scientific reviewJAMA2002287788388911851582
- MeijlerFLSir James Black, FRS, FRCP, FACC: Nobel laureate 1988J Am Coll Cardiol19891337697702537350
- HerleniusELagercrantzHNeurotransmitters and neuromodulators during early human developmentEarly Hum Dev2001651213711520626
- Anitole-MislehKGBrownKMDevelopmental regulation of catecholamine levels during sea urchin embryo morphogenesisComp Biochem Physiol A Mol Integr Physiol20041371395014720589
- KimMONaSILeeMYHeoJSHanHJEpinephrine increases DNA synthesis via ERK1/2s through cAMP, Ca(2+)/PKC, and PI3K/Akt signaling pathways in mouse embryonic stem cellsJ Cell Biochem200810441407142018275042
- SloanEKPricemanSJCoxBFThe sympathetic nervous system induces a metastatic switch in primary breast cancerCancer Res201070187042705220823155
- HanceMWDharMSPlummerHK3rdG-protein inwardly rectifying potassium channel 1 (GIRK1) knockdown decreases beta-adrenergic, MAP kinase and Akt signaling in the MDA-MB-453 breast cancer cell lineBreast Cancer (Auckl)20081253421655370
- Al-WadeiHAAl-WadeiMHSchullerHMCooperative regulation of non-small cell lung carcinoma by nicotinic and beta-adrenergic receptors: a novel target for interventionPLoS One201271e2991522253823
- HuangXYWangHCYuanZHuangJZhengQNorepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via beta-adrenergic receptor-dependent activation of P38/MAPK pathwayHepatogastroenterology20125911588989322020907
- Al-WadeiHAPlummerHK3rdSchullerHMNicotine stimulates pancreatic cancer xenografts by systemic increase in stress neurotransmitters and suppression of the inhibitory neurotransmitter gamma-aminobutyric acidCarcinogenesis200930350651119131543
- ZhangDMaQWangZBeta2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFkappaB pathwayMol Cancer20111014622118662
- PalmDLangKNiggemannBThe norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockersInt J Cancer2006118112744274916381019
- HazutOShaashuaLBenishMThe effect of beta-adrenergic blockade and COX-2 inhibition on healing of colon, muscle, and skin in rats undergoing colonic anastomosisInt J Clin Pharmacol Ther201149954555421888867
- ShinVYJinHCNgEKNicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone induce cyclooxygenase-2 activity in human gastric cancer cells: Involvement of nicotinic acetylcholine receptor (nAChR) and beta-adrenergic receptor signaling pathwaysToxicol Appl Pharmacol2008233225426118805435
- LeeJWShahzadMMLinYGSurgical stress promotes tumor growth in ovarian carcinomaClin Cancer Res20091582695270219351748
- SchullerHMIs cancer triggered by altered signalling of nicotinic acetylcholine receptors?Nat Rev Cancer20099319520519194381
- MontminyMTranscriptional regulation by cyclic AMPAnnu Rev Biochem1997668078229242925
- ZhangXOdomDTKooSHGenome-wide analysis of cAMP-response element binding protein occupancy, phosphorylation, and target gene activation in human tissuesProc Natl Acad Sci U S A2005102124459446415753290
- LuttrellLMFergusonSSDaakaYBeta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexesScience199928354026556619924018
- de RooijJZwartkruisFJVerheijenMHEpac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMPNature199839667104744779853756
- ZiebaBJArtamonovMVJinLThe cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activityJ Biol Chem201128619166811669221454546
- ShahzadMMArevaloJMArmaiz-PenaGNStress effects on FosB- and interleukin-8 (IL8)-driven ovarian cancer growth and metastasisJ Biol Chem201028546354623547020826776
- BernabeDGTamaeACBiasoliEROliveiraSHStress hormones increase cell proliferation and regulates interleukin-6 secretion in human oral squamous cell carcinoma cellsBrain Behav Immun201125357458321187140
- ColeSWArevaloJMTakahashiRComputational identification of gene-social environment interaction at the human IL6 locusProc Natl Acad Sci U S A2010107125681568620176930
- ChakrobortyDSarkarCBasuBDasguptaPSBasuSCatecholamines regulate tumor angiogenesisCancer Res20096993727373019383906
- ThakerPHHanLYKamatAAChronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinomaNat Med200612893994416862152
- EntschladenFDrellTTLangKJosephJZaenkerKSTumour-cell migration, invasion, and metastasis: navigation by neurotransmittersLancet Oncol20045425425815050959
- SoodAKBhattyRKamatAAStress hormone-mediated invasion of ovarian cancer cellsClin Cancer Res200612236937516428474
- JinZGaoFFlaggTDengXNicotine induces multi-site phosphorylation of Bad in association with suppression of apoptosisJ Biol Chem200427922238372384415037618
- SoodAKArmaiz-PenaGNHalderJAdrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikisJ Clin Invest201012051515152320389021
- HaraMRKovacsJJWhalenEJA stress response pathway regulates DNA damage through beta2-adrenoreceptors and beta-arrestin-1Nature2011477736434935321857681
- SacharczukMJaszczakKSadowskiBChromosomal NOR activity in mice selected for high and low swim stress-induced analgesiaBehav Genet200333443544114574142
- GlaserRKiecolt-GlaserJKStress-induced immune dysfunction: implications for healthNat Rev Immunol20055324325115738954
- AntoniMHLutgendorfSKColeSWThe influence of bio-behavioural factors on tumour biology: pathways and mechanismsNat Rev Cancer20066324024816498446
- GoldfarbYSorskiLBenishMLeviBMelamedRBen-EliyahuSImproving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responsesAnn Surg2011253479881021475023
- GlasnerAAvrahamRRosenneEImproving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitorJ Immunol201018452449245720124103
- GreenbergerSBoscoloEAdiniIMullikenJBBischoffJCorticosteroid suppression of VEGF-A in infantile hemangioma-derived stem cellsN Engl J Med2010362111005101320237346
- AsaharaTTakahashiTMasudaHVEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cellsEMBO J199918143964397210406801
- KwakCJinRJLeeCParkMSLeeSEThrombospondin-1, vascular endothelial growth factor expression and their relationship with p53 status in prostate cancer and benign prostatic hyperplasiaBJU Int200289330330911856116
- ToiMInadaKSuzukiHTominagaTTumor angiogenesis in breast cancer: its importance as a prognostic indicator and the association with vascular endothelial growth factor expressionBreast Cancer Res Treat19953621932048534867
- El-AssalONYamanoiASodaYClinical significance of microvessel density and vascular endothelial growth factor expression in hepatocellular carcinoma and surrounding liver: possible involvement of vascular endothelial growth factor in the angiogenesis of cirrhotic liverHepatology1998276155415629620326
- ZhangLLinXWangWCirculating level of vascular endothelial growth factor in differentiating hemangioma from vascular malformation patientsPlast Reconstr Surg2005116120020415988268
- ParkSYKangJHJeongKJNorepinephrine induces VEGF expression and angiogenesis by a hypoxia-inducible factor-1alpha protein-dependent mechanismInt J Cancer2011128102306231620715173
- IaccarinoGCiccarelliMSorrientoDIschemic neoangiogenesis enhanced by beta2-adrenergic receptor overexpression: a novel role for the endothelial adrenergic systemCirc Res200597111182118916239589
- CiccarelliMSorrientoDCipollettaEImpaired neoangiogenesis in beta(2)-adrenoceptor gene-deficient mice: restoration by intravascular human beta(2)-adrenoceptor gene transfer and role of NFkappaB and CREB transcription factorsBr J Pharmacol2011162371272120958287
- Léauté-LabrèzeCDumasDLREHubicheTBoraleviFThamboJBTaiebAPropranolol for severe hemangiomas of infancyN Engl J Med2008358242649265118550886
- StorchCHHoegerPHPropranolol for infantile haemangiomas: insights into the molecular mechanisms of actionBr J Dermatol2010163226927420456345
- ChimHArmijoBSMillerEGliniakCSerretMAGosainAKPropranolol induces regression of hemangioma cells through HIF-1alpha-mediated inhibition of VEGF-AAnn Surg2012256114615622580939
- ColeSWSoodAKMolecular pathways: beta-adrenergic signaling in cancerClin Cancer Res20121851201120622186256
- SchullerHMBeta-adrenergic signaling, a novel target for cancer therapy?Oncotarget20101746646921317444
- SchullerHMColeBRegulation of cell proliferation by beta-adrenergic receptors in a human lung adenocarcinoma cell lineCarcinogenesis1989109175317552569945
- Al-WadeiHATakahashiTSchullerHMCaffeine stimulates the proliferation of human lung adenocarcinoma cells and small airway epithelial cells via activation of PKA, CREB and ERK1/2Oncol Rep200615243143516391865
- Al-WadeiHAUllahMFAl-WadeiMHIntercepting neoplastic progression in lung malignancies via the beta adrenergic (beta-AR) pathway: implications for anti-cancer drug targetsPharmacol Res2012661334022487140
- LiuXWuWKYuLEpinephrine stimulates esophageal squamous-cell carcinoma cell proliferation via beta-adrenoceptor-dependent transactivation of extracellular signal-regulated kinase/cyclooxygenase-2 pathwayJ Cell Biochem20081051536018452159
- SlotkinTAZhangJDancelRGarciaSJWillisCSeidlerFJBeta-adrenoceptor signaling and its control of cell replication in MDA-MB-231 human breast cancer cellsBreast Cancer Res Treat200060215316610845278
- CarieAESebtiSMA chemical biology approach identifies a beta-2 adrenergic receptor agonist that causes human tumor regression by blocking the Raf-1/Mek-1/Erk1/2 pathwayOncogene200726263777378817260025
- SchullerHMTithofPKWilliamsMPlummerHRThe tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone is a beta-adrenergic agonist and stimulates DNA synthesis in lung adenocarcinoma via beta-adrenergic receptor-mediated release of arachidonic acidCancer Res199959184510451510493497
- SchullerHMOrloffMTobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cellsBiochem Pharmacol19985591377138410076528
- SchullerHMWitschiHPNylenEJoshiPACorreaEBeckerKLPathobiology of lung tumors induced in hamsters by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and the modulating effect of hyperoxiaCancer Res1990506196019652306745
- LaagEMajidiMCekanovaMMasiTTakahashiTSchullerHMNNK activates ERK1/2 and CREB/ATF-1 via beta-1-AR and EGFR signaling in human lung adenocarcinoma and small airway epithelial cellsInt J Cancer200611971547155216671086
- MajidiMAl-WadeiHATakahashiTSchullerHMNongenomic beta estrogen receptors enhance beta1 adrenergic signaling induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in human small airway epithelial cellsCancer Res200767146863687117638897
- WongHPYuLLamEKTaiEKWuWKChoCHNicotine promotes colon tumor growth and angiogenesis through beta-adrenergic activationToxicol Sci200797227928717369603
- WongHPYuLLamEKTaiEKWuWKChoCHNicotine promotes cell proliferation via alpha7-nicotinic acetylcholine receptor and catecholamine-synthesizing enzymes-mediated pathway in human colon adenocarcinoma HT-29 cellsToxicol Appl Pharmacol2007221326126717498763
- PierceKLPremontRTLefkowitzRJSeven-transmembrane receptorsNat Rev Mol Cell Biol20023963965012209124
- JiYLiKXiaoXZhengSXuTChenSEffects of propranolol on the proliferation and apoptosis of hemangioma-derived endothelial cellsJ Pediatr Surg2012
- SastryKSKarpovaYProkopovichSEpinephrine protects cancer cells from apoptosis via activation of cAMP-dependent protein kinase and BAD phosphorylationJ Biol Chem200728219140941410017353197
- ZhangDMaQShenSHuHInhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cellPancreas20093819410019106745
- ShanTMaQZhangDBeta2-adrenoceptor blocker synergizes with gemcitabine to inhibit the proliferation of pancreatic cancer cells via apoptosis inductionEur J Pharmacol20116651–31721570961
- LiaoXCheXZhaoWZhangDBiTWangGThe beta-adrenoceptor antagonist, propranolol, induces human gastric cancer cell apoptosis and cell cycle arrest via inhibiting nuclear factor kappaB signalingOncol Rep20102461669167621042766
- YawataAAdachiMOkudaHProlonged cell survival enhances peritoneal dissemination of gastric cancer cellsOncogene19981620268126869632144
- SoodAKArmaiz-PenaGNHalderJAdrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikisJ Clin Invest201012051515152320389021
- MarxJCancer research. Inflammation and cancer: the link grows strongerScience2004306569896696815528423
- XieGYaoQLiuYIL-6-induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere culturesInt J Oncol20124041171117922134360
- ObataNHTamakoshiKShibataKKikkawaFTomodaYEffects of interleukin-6 on in vitro cell attachment, migration and invasion of human ovarian carcinomaAnticancer Res1997171A3373429066674
- HeikkilaKEbrahimSLawlorDASystematic review of the association between circulating interleukin-6 (IL-6) and cancerEur J Cancer200844793794518387296
- ScambiaGTestaUPaniciPBInterleukin-6 serum levels in patients with gynecological tumorsInt J Cancer19945733183238168990
- RohrbachSEngelhardtSLohseMJWerdanKHoltzJMuller-WerdanUActivation of AP-1 contributes to the beta-adrenoceptor-mediated myocardial induction of interleukin-6Mol Med20071311–1260561417948064
- YangEVKimSJDonovanELNorepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progressionBrain Behav Immun200923226727518996182
- TanKSNackleyAGSatterfieldKMaixnerWDiatchenkoLFloodPMBeta2 adrenergic receptor activation stimulates pro-inflammatory cytokine production in macrophages via PKA- and NF-kappaB-independent mechanismsCell Signal200719225126016996249
- NilssonMBArmaiz-PenaGTakahashiRStress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanismJ Biol Chem200728241299192992617716980
- MaddenKSSzpunarMJBrownEBBeta-adrenergic receptors (beta-AR) regulate VEGF and IL-6 production by divergent pathways in high beta-AR-expressing breast cancer cell linesBreast Cancer Res Treat2011130374775821234673
- KochAEPolveriniPJKunkelSLInterleukin-8 as a macrophage-derived mediator of angiogenesisScience19922585089179818011281554
- KassimSKEl-SalahyEMFayedSTVascular endothelial growth factor and interleukin-8 are associated with poor prognosis in epithelial ovarian cancer patientsClin Biochem200437536336915087251
- SchreiberRDOldLJSmythMJCancer immunoediting: integrating immunity’s roles in cancer suppression and promotionScience201133160241565157021436444
- GoldfarbYSorskiLBenishMLeviBMelamedRBen-EliyahuSImproving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responsesAnn Surg2011253479881021475023
- McGregorBAAntoniMHBoyersAAlferiSMBlombergBBCarverCSCognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancerJ Psychosom Res20045611814987957
- AndersenBLFarrarWBGolden-KreutzDMPsychological, behavioral, and immune changes after a psychological intervention: a clinical trialJ Clin Oncol200422173570358015337807
- CunnickJELysleDTKucinskiBJRabinBSEvidence that shock-induced immune suppression is mediated by adrenal hormones and peripheral beta-adrenergic receptorsPharmacol Biochem Behav19903636456512165621
- PollardJWTumour-educated macrophages promote tumour progression and metastasisNat Rev Cancer200441717814708027
- MasurKNiggemannBZankerKSEntschladenFNorepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockersCancer Res20016172866286911306460
- LangKDrellTTLindeckeAInduction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugsInt J Cancer2004112223123815352035
- DrellTTJosephJLangKNiggemannBZaenkerKSEntschladenFEffects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cellsBreast Cancer Res Treat2003801637012889599
- JosephJNiggemannBZaenkerKSEntschladenFThe neurotransmitter gamma-aminobutyric acid is an inhibitory regulator for the migration of SW 480 colon carcinoma cellsCancer Res200262226467646912438237
- YangEVBaneCMMacCallumRCKiecolt-GlaserJKMalarkeyWBGlaserRStress-related modulation of matrix metalloproteinase expressionJ Neuroimmunol20021331–214415012446017
- EntschladenFDrellTTLangKJosephJZaenkerKSTumour-cell migration, invasion, and metastasis: navigation by neurotransmittersLancet Oncol20045425425815050959
- LutgendorfSKLamkinDMJenningsNBBiobehavioral influences on matrix metalloproteinase expression in ovarian carcinomaClin Cancer Res200814216839684618980978
- LandenCJLinYGArmaizPGNeuroendocrine modulation of signal transducer and activator of transcription-3 in ovarian cancerCancer Res20076721103891039617974982
- YangEVSoodAKChenMNorepinephrine up-regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)-2, and MMP-9 in nasopharyngeal carcinoma tumor cellsCancer Res20066621103571036417079456
- GuoKMaQWangLNorepinephrine-induced invasion by pancreatic cancer cells is inhibited by propranololOncol Rep200922482583019724861
- SchullerHMAl-WadeiHAUllahMFPlummerHRRegulation of pancreatic cancer by neuropsychological stress responses: a novel target for interventionCarcinogenesis201233119119622072614
- Al-WadeiHAPlummerHRUllahMFUngerBBrodyJRSchullerHMSocial stress promotes and gamma-aminobutyric acid inhibits tumor growth in mouse models of non-small cell lung cancerCancer Prev Res (Phila)20125218919621955519
- PoweDGEntschladenFTargeted therapies: Using beta-blockers to inhibit breast cancer progressionNat Rev Clin Oncol20118951151221808268
- [No authors listed]Medical Research Council trial of treatment of hypertension in older adults: principal results. MRC Working PartyBMJ199230468244054121445513
- JickHCalcium-channel blockers and risk of cancerLancet19973499066169917009186407
- AlgaziMPlu-BureauGFlahaultADondonMLeMGIs beta-blocker treatment associated with a decrease in the risk of cancerLett Drug Des Discov200693653661
- PerronLBairatiIHarelFMeyerFAntihypertensive drug use and the risk of prostate cancer (Canada)Cancer Causes Control200415653554115280632
- FitzpatrickALDalingJRFurbergCDKronmalRAWeissfeldJLHypertension, heart rate, use of antihypertensives, and incident prostate cancerAnn Epidemiol201111853454211709272
- AssimesTLElsteinELanglebenASuissaSLong-term use of antihypertensive drugs and risk of cancerPharmacoepidemiol Drug Saf200817111039104918780400
- GrossmanEMesserliFHGoldbourtUAntihypertensive therapy and the risk of malignanciesEur Heart J200122151343135211465967
- JansenLBelowJChang-ClaudeJBrennerHHoffmeisterMBeta blocker use and colorectal cancer risk: population-based case-control studyCancer2012118163911391922585669
- RodriguezCJacobsEJDekaAUse of blood-pressure-lowering medication and risk of prostate cancer in the Cancer Prevention Study II Nutrition CohortCancer Causes Control200920567167919067188
- De GiorgiVGrazziniMGandiniSTreatment with beta-blockers and reduced disease progression in patients with thick melanomaArch Intern Med2011171877978121518948
- LemeshowSSorensenHTPhillipsGBeta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort studyCancer Epidemiol Biomarkers Prev201120102273227921933972
- BarronTIConnollyRMSharpLBennettKVisvanathanKBeta blockers and breast cancer mortality: a population- based studyJ Clin Oncol201129192635264421632503
- Melhem-BertrandtAChavez-MacgregorMLeiXBeta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancerJ Clin Oncol201129192645265221632501
- BangaloreSKumarSKjeldsenSEAntihypertensive drugs and risk of cancer: network meta-analyses and trial sequential analyses of 324,168 participants from randomised trialsLancet Oncol2011121658221123111
- PoweDGVossMJZankerKSBeta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survivalOncotarget20101762863821317458
- GanzPAHabelLAWeltzienEKCaanBJColeSWExamining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohortBreast Cancer Res Treat2011129254955621479924
- SansVde la RoqueEDBergeJPropranolol for severe infantile hemangiomas: follow-up reportPediatrics20091243e423e43119706583
- BigorreMVan KienAKValetteHBeta-blocking agent for treatment of infantile hemangiomaPlast Reconstr Surg20091236195e196e
- LeboulangerNFayouxPTeissierNPropranolol in the therapeutic strategy of infantile laryngotracheal hemangioma: A preliminary retrospective study of French experienceInt J Pediatr Otorhinolaryngol201074111254125720800295
- RaphaelMFde GraafMBreugemCCPasmansSGBreurJMAtenolol: a promising alternative to propranolol for the treatment of hemangiomasJ Am Acad Dermatol201165242042121763565
- ChakkittakandiyilAPhillipsRFriedenIJTimolol maleate 0.5% or 0.1% gel-forming solution for infantile hemangiomas: a retrospective, multicenter, cohort studyPediatr Dermatol2012291283122150436
- HogelingMAdamsSWargonOA randomized controlled trial of propranolol for infantile hemangiomasPediatrics20111282e259e26621788220
- PopeEChakkittakandiyilALara-CorralesIMakiEWeinsteinMExpanding the therapeutic repertoire of infantile hemangiomas: Cohort blinded study of oral nadolol compared with propranololBr J Dermatol2012
- PierceBLBallard-BarbashRBernsteinLElevated biomarkers of inflammation are associated with reduced survival among breast cancer patientsJ Clin Oncol200927213437344419470939
- Ben-EliyahuSShakharGPageGGStefanskiVShakharKSuppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptorsNeuroimmunomodulation20008315416411124582
- SmithCTeitlerMBeta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptorsCardiovasc Drugs Ther199913212312610372227
- GanzPAColeSWExpanding our therapeutic options: beta blockers for breast cancer?J Clin Oncol201129192612261621632500