Abstract
The co-occurrence of a vestibular schwannoma and a low-grade glioma is rare, and even rarer is the association with an oligodendroglioma. Although various authors have addressed the problem of treating patients with incidentally discovered indolent low-grade gliomas, an established protocol does not exist to date. The common approach is to reserve surgery until there is radiological evidence of tumor growth or high-grade transformation. However, because incidental low-grade glioma may represent the first stage of unavoidable pathological progression towards high-grade glioma, early and radical surgical resection should be advocated in order to increase the chance of a “cure” and prolonged survival. This case report supports this view, and suggests reflection on a possible change from a conservative philosophy to preventative surgical treatment.
Introduction
Multiple primary intracranial tumors of different histological types are rare, except for cases observed after radiotherapy or in phacomatosis. This report concerns a patient with a left vestibular schwannoma and an incidentally discovered contralateral oligodendroglioma which was operated on 5 years later for tumor progression. The association between vestibular schwannomas and low-grade gliomas has been reported only a few times in the literature and no report specifically refers to oligodendroglioma.Citation1,Citation2 Symptomatic low-grade gliomas usually debut with new-onset seizures, and their prompt treatment is advisable for oncological and functional reasons. On the other hand, it is still unclear how to best manage these lesions when they present as an incidental finding during brain imaging for unrelated complaints or research studies.Citation3,Citation4
Case report
A 43-year-old woman was admitted to our department with a 16-month history of progressive left-sided hearing loss, tinnitus, and disequilibrium. Audiometry revealed mild sensorineural hearing loss in the left ear (American Academy of Otolaryngology Head and Neck Surgery class B). Magnetic resonance imaging (MRI) showed a large extra-axial mass within the left cerebellopontine angle, extending into the left internal auditory canal with a marked compressive effect on the upper brainstem. The lesion appeared hyperintense on T2 and hypointense on T1 sequences, with homogeneous enhancement after gadolinium administration, suggesting a diagnosis of vestibular schwannoma ( and ). MRI also revealed an incidental right insular lesion with high signal intensity on T2-weighted images and low signal intensity on T1-weighted images. No contrast enhancement was detected. MRI features were suggestive of a low-grade glioma ( and ). The patient showed no signs of phacomatosis and a genetics screen was negative, as was her family history.
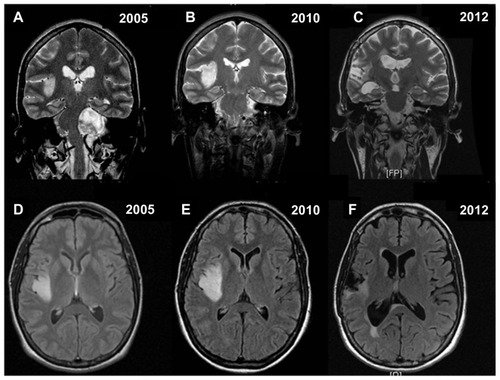
Figure 1 Contrast-enhanced coronal and axial T1-weighted magnetic resonance images showing the synchronous association of a left vestibular schwannoma and a right insular low grade glioma located in insular zone II–III according to the Berger–Sanai classification system (A and D). Five years later, magnetic resonance imaging documenting removal of vestibular schwannoma, as well as growth and possible high-grade transformation, because of the presence of focal areas of enhancement, of the right insular low-grade glioma (B and E). Postoperative final magnetic resonance imaging showing satisfactory removal of the insular oligodendroglioma (World Health Organization grade III), with only a small posteromedial residual tumor adjacent to the internal capsule, and stability of the left intrameatal vestibular schwannoma (C and F).
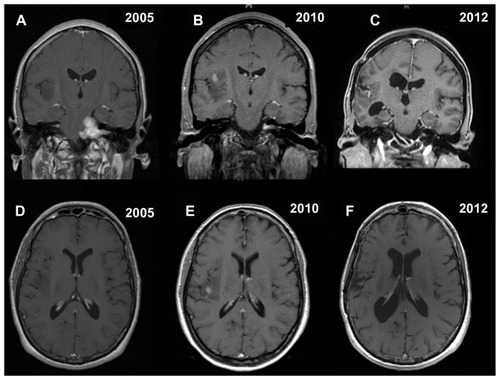
Figure 2 Coronal T2-weighted and axial flair magnetic resonance images confirming the findings of (A–F).
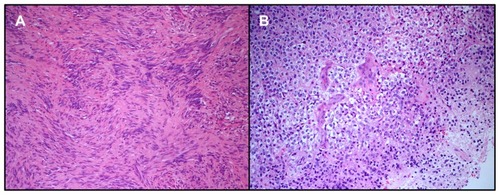
She underwent surgery using a suboccipital retrosigmoid approach, which allowed near-total gross resection of the tumor, with only a small residual intrameatal component, decompression of the brainstem, and preservation of VII and VIII cranial nerve function ( and ). Her postoperative course was uneventful. Histopathological examination demonstrated the presence of spindle cells with elongated nuclei arranged in bundles in various directions and occasional small vessels. The tumor showed intense and diffuse staining for S100 protein. The diagnosis was vestibular schwannoma ().
Figure 3 Loosely textured neoplasm made of spindle cells without significant cytological atypia seen on hematoxylin and eosin staining. Mitotic figures were not seen. The lesion was intensively stained by S100 protein (not shown). This image was taken from a small area of the tumor where the nuclei were aligned, suggesting initial formation of Verocay bodies. No true Verocay bodies were found since the lesion had mostly regressive areas. The morphology is consistent with a diagnosis of schwannoma (A). Hematoxylin and eosin staining showing an oligodendroglioma made of monomorphic cells with rounded nuclei and clear perinuclear halos. This image shows a small area of necrosis and newly formed blood vessels, consistent with early anaplastic transformation in the oligodendroglioma (B).
MRI performed 3 months after surgery then every 9 months thereafter confirmed a residual of the cerebellopontine angle tumor in the left internal acoustic meatus, which remains stable to date. MRI also allowed close monitoring of the asymptomatic right insular lesion, which showed no changes on neuroimaging follow-up for about 5 years after surgery.
The patient was then hospitalized again for sudden onset of partial motor seizures in the left hemisoma. MRI at this time showed slight growth of the right temporal lesion with focal areas of contrast enhancement suggestive of high-grade transformation. These new clinical and neuroradiological findings prompted us to plan tumor resection assisted by intraoperative monitoring and brain mapping via a right frontotemporal craniotomy and a transsylvian approach. Postoperative MRI documented total gross resection of the lesion ( and ). The postoperative course was uneventful, and the patient was discharged 5 days after surgery. The sample was characterized histologically by mildly enlarged cells infiltrating normal brain parenchyma and producing vague nodules separated by a dense network of branching capillaries. The cells had round homogenous nuclei with delicate chromatin and a swollen clear cytoplasm. Focal areas with higher cellularity, nuclear atypia, mitoses, necrosis, and newly formed blood vessels were also present. The proliferative rate in these areas was about 15%–20%. Fluorescence in situ hybridization analysis revealed a 1p/19q codeletion. Isocitrate dehydrogenase status was tested by immunohistochemistry. The majority of the nuclei were positive, allowing for demonstration of the mutation. The diagnosis was low-grade oligodendroglioma (World Health Organization grade II) with focal areas of grade III anaplasia ().
The patient was treated with adjuvant fractionated radiotherapy (focal irradiation in daily fractions of 2 Gy given 5 days per week for 6 weeks, for a total of 60 Gy) and subsequent chemotherapy with temozolomide 150 mg/m2/day on days 1–5 of a 28-day cycle, and increased to 200 mg/m2/day for the following cycles. Eighteen temozolomide cycles have been administered to date, and the patient, after almost 2 years of follow-up, is seizure-free with a Karnofsky performance score of 100. Levetiracetam 500 mg twice daily is also being administered. The most recent follow-up MRI confirmed no regrowth of the small posteromedial remnant tumor ( and ). The intention is to give the patient up to 24 cycles of temozolomide, in the absence of disease progression or unacceptable toxicity.
Discussion
This case has a dual oncological interest, ie, the rare cooccurrence of two histologically unrelated brain tumors and the difficult decision-making process concerning the best treatment for a patient with an asymptomatic incidental lesion and concurrent central nervous system disease. For the case reported here, controversy centered around the optimal timing of surgical treatment for an indolent low-grade glioma.
Vestibular schwannomas originate in the Schwann cells of the vestibular branch of the auditory nerve, while oligodendrogliomas are believed to derive from progenitor oligodendrocytes. The similarity between the two groups of cells is that both are lining and supporting cells for neurons.Citation5,Citation6 Except for cases observed after radiotherapy or in phacomatosis, multiple primary intracranial tumors of different histological types are rare. Several mechanisms have been proposed, but there is no conclusive evidence for any of these. Based on the observation of adjacent tumoral localization in a large percentage of reported cases, it was thought that one tumor may act as an irritative stimulus for the development of the other. Some authors suggest a common carcinogenic agent, whereas others believe that such tumor combinations occur simply by chance.Citation1 The incidence of vestibular schwannomas is increased in neurofibromatosis, where they tend to co-occur with other intracranial tumors, such as gliomas or meningiomas. However, only few cases of simultaneous tumors have been reported in patients without neurofibromatosis, and the association of vestibular schwannoma with glioma is very rare.Citation1,Citation2
In the past, multiple brain tumors were detected only at autopsy, but nowadays, with the rapid development of noninvasive neuroimaging (computed tomography and MRI), an increasing number of incidental lesions are being detected during investigations for unrelated complaints, eg, headache or traumatic brain injury.Citation2–Citation4 With regard to incidental low-grade gliomas, data from the French Brain Tumor Databank have reported an incidence of 3.0%.Citation7 Olson et alCitation8 observed an incidence of 4.7%, Kamiguchi et alCitation9 reported an incidence of 4.9%, and Pallud et alCitation2 described 47 asymptomatic low-grade gliomas from 1296 World Health Organization grade II gliomas (3.8%). Therefore, in this new setting, while there is general agreement that symptomatic low-grade gliomas should be treated immediately, several concerns remain about the best management for incidental asymptomatic ones, especially regarding the timing of surgical treatment. In fact, although several authors have addressed the problem of treating patients with incidental low-grade gliomas, an established protocol does not exist to date. The common attitude is that surgery is indicated when there is radiological evidence of growth or signs of high-grade transformation,Citation2,Citation3 considering the relatively elevated surgical risk in asymptomatic and usually young patients. However, there is recent good evidence suggesting a need to consider a shift from this conservative philosophy to more preventive surgical neurooncology.Citation10 Several authors have suggested that quiescent low-grade gliomas do not exist, and that all gliomas will progress. The estimated mean time for indolent low-grade gliomas to become symptomatic is 4 years, and around 5 years for high-grade transformation.Citation2,Citation4,Citation11 Hence, incidental low-grade gliomas may be the first stage in pathological progression towards high-grade gliomas. Duffau reported that a microfocus with endothelial proliferation was identified in the middle of the tumor in 27% of operated indolent low-grade gliomas, indicating that high-grade transformation may precociously occur.Citation12 These data suggest that even small indolent low-grade gliomas are unstable and not benign lesions, in which preventative and potentially curative surgery is advisable in order to maximize the extent of resection before uncontrollable glioma growth and migration happens. At the same time, early surgery prevents unavoidable progression with the risk of anaplastic transformation which ultimately leads to the deaths of affected patients. Hence, maximal resection is currently considered the first therapeutic option for low-grade gliomas,Citation13,Citation14 and this has also recommended in the European Association of Neurooncology guidelines for management of low-grade gliomas.Citation15
Regarding the safety of surgery, several recent reports have documented its feasibility and a positive balance between maximal extent of resection and relative functional risk, even in eloquent areas of the brain, as a result of extensive development of preoperative functional studies and intraoperative tools, including endoscopy, fluorescein angiography, advanced intraoperative imaging, and brain mapping.Citation10,Citation16
In conclusion, the present case report reinforces the philosophy of early surgical treatment, even in eloquent areas, such as zone II–III of the Berger–Sanai classification system where the insular glioma was located, because the patient inevitably became symptomatic and showed high-grade tumor transformation within a few years. Conversely, radical resection of low-grade gliomas is advocated, because it can be safe and efficacious, enabling, as already mentioned, significant delay in tumor progression and a consequent increase in overall survival.Citation10,Citation16–Citation18
Conclusion
Vestibular schwannomas have been reported only a few times in association with a synchronous low-grade glioma, but never, to the best of our knowledge, specifically with an oligodendroglioma. The best therapeutic option for the so-called “indolent” low-grade glioma is still unclear. However, these tumors are unstable and not benign lesions, which present unavoidably with high-grade transformation, as happened in the case reported here. Therefore, early preventative surgical treatment is preferable to the common “wait-and-see” approach.
Disclosure
The authors report no conflicts of interest in this work.
References
- ButtiGGiordanaMTPaolettiPSchifferDMultiple primary intracranial tumors of different cell types: association of anaplastic astrocytoma and acoustic neurinoma – with review of the literatureSurg Neurol19821853363427179095
- PalludJFontaineDDuffauHNatural history of incidental World Health Organization grade II gliomasAnn Neurol201068572773321031584
- ShahAHMadhavanKHerosDThe management of incidental low-grade gliomas using magnetic resonance imaging: systematic review and optimal treatment paradigmNeurosurg Focus2011316E1222133169
- PottsMBSmithJSMolinaroAMBergerMSNatural history and surgical management of incidentally discovered low-grade gliomasJ Neurosurg2012116236537221999317
- EngelhardHHSteleaACochranEJOligodendroglioma: pathology and molecular biologySurg Neurol200258211111712453646
- MatthiesCSamiiMAcoustic neuromas (vestibular schwannomas)BergerMSPradosMTextbook of Neurooncology1st edPhiladelphia, PASaunders2004
- BauchetLRigauVMathieu-DaudeHFrench brain tumor data bank: methodology and first results on 10,000 casesJ Neurooncol200784218919917431547
- OlsonJDRiedelEDeAngelisLMLong-term outcome of lowgrade oligodendroglioma and mixed gliomaNeurology20005471442144810751254
- KamiguchiHShiobaraRToyaSAccidentally detected brain tumors: clinical analysis of a series of 110 patientsClin Neurol Neurosurg19969821711758836593
- DuffauHThe rationale to perform early resection in incidental diffuse low-grade glioma: toward a “preventive surgical neurooncology”World Neurosurg
- PalludJTaillandierLCapelleLQuantitative morphological magnetic resonance imaging follow-up of low-grade glioma: a plea for systematic measurement of growth ratesNeurosurgery201271372973922668885
- DuffauHAwake surgery for incidental WHO grade II gliomas involving eloquent areasActa Neurochir (Wien)2012154457558422139145
- DuffauHSurgery of low-grade gliomas: towards a ‘functional neurooncology’Curr Opin Oncol200921654354919606033
- SmithJSChangEFLambornKRRole of extent of resection in the long-term outcome of low-grade hemispheric gliomasJ Clin Oncol20082681338134518323558
- SoffiettiRBaumertBGBelloLGuidelines on management of low-grade gliomas: report of an EFNS-EANO Task ForceEur J Neurol20101791124113320718851
- SanaiNPolleyMYBergerMSInsular glioma resection: assessment of patient morbidity, survival, and tumor progressionJ Neurosurg201011211919612970
- ScerratiMRoselliRIacoangeliMPompucciARossiGFPrognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: the role of surgeryJ Neurol Neurosurg Psychiatry19966132912968795601
- IacoangeliMRoselliRPreziosoAScerratiMRossiGFStaging of supratentorial hemispheric glioma using tumour extension, histopathological grade and extent of surgical resectionBr J Surg1993809113011338402111