Abstract
Preclinical work has led to an increased understanding of the immunomodulatory mechanisms involved in the regulation of the antitumor response in a variety of tumor types. PD-1 (programmed death 1) appears to be a key checkpoint involved in immune suppression in the tumor microenvironment, even in diseases not previously thought to be sensitive to immune manipulation. More recently, the subsequent clinical development of PD-1-based therapy has resulted in a major breakthrough in the field of oncology. Pembrolizumab, a humanized highly selective IgG4 anti-PD-1 monoclonal antibody, was recently approved for the treatment of advanced melanoma based on promising early-phase clinical data. Encouraging results have also been seen in other malignancies, and PD-1-targeted therapies are likely to markedly change the treatment landscape. Future work will center on rationally designed combination strategies in order to potentiate the antitumor immune response and overcome mechanisms of resistance.
Introduction
Immune-directed therapies have recently demonstrated improved efficacy in several tumor types, with significant advances resulting from the development of PD-1 (programmed death 1)-blocking agents, including pembrolizumab (formerly MK-3475). Drugs with immune-mediated activity have been used for decades in oncology, with high-dose interleukin-2 (IL-2) having been approved for metastatic melanoma in 1998 and adjuvant interferon-α2B approved in 1995.Citation1,Citation2 More recently, the CTLA-4 blocking agent, ipilimumab, and the vaccine therapy sipuleucel-T have gained approvals as well; however, while a small number of patients do gain long-lasting clinical benefit with each of these agents, the overall survival benefit in all patients treated with these agents remains small and the potential toxicity is significant.Citation3,Citation4
The initial reports of clinical activity with PD-1 blockade using novel investigational agents demonstrated the feasibility of high rates of durable responses with immune therapy in several tumor types, in addition to melanoma and renal cell carcinoma (RCC), which had long been considered to be potentially immune responsive. The great potential for clinical activity with highly effective immune-directed therapy across many tumor types has now been realized; several ongoing and recently completed clinical trials have focused on developing safe and effective single-agent and combination immune therapy strategies, including PD-1 blockade. The newly realized possibilities of long-term disease control, improved survival, and, perhaps, even cure with effective immune-directed therapies highlight the importance of modulating the immune system in the care of oncology patients.
Here we review the clinical development of PD-1-based therapy, with a particular focus on the PD-1-blocking agent, pembrolizumab, in the treatment of advanced cancer.
Mode of action and pharmacology of pembrolizumab
The PD-1 pathway is an immune regulatory mechanism (). The discovery of PD-1 blockade as a potential mechanism of T-cell activation and antitumor activity was originally based on studies of T cells in chronic infection models. Activated T cells (defined by the expression of activation markers CD69hi, CD44hi, and CD62Llo) which were lacking effector function were initially described as key mediators underlying the mechanism of viral immune evasion.Citation5 In the presence of chronic viral infection, CD8 T cells develop a dysfunctional or exhausted T-cell phenotype characterized by a higher expression of PD-1 compared to functional T cells. Functional T cells may transiently express negative regulatory molecules, including PD-1, in states of activation; however, persistently high expression indicates an exhausted and dysfunctional T-cell phenotype.Citation6,Citation7 Of note, blockade of the PD-1 and ligand (PD-L1) interaction in a mouse model of chronic infection could restore T-cell function, with a resulting reduction in viral load.Citation8 Subsequent studies identified the same pattern of provoked T-cell dysfunction as a mechanism of tumor evasion of the immune system, leading to the development of therapeutic PD-1 blockade in patients with advanced malignancies.Citation9–Citation12
Figure 1 Overview of the PD-1 pathway.
Abbreviations: MHC, major histocompatibility complex; TCR, T-cell receptor; PD-1, programmed death 1; PD-L1, PD-1 ligand.
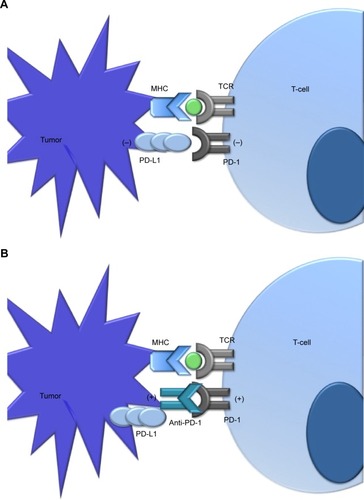
Pembrolizumab is a humanized PD-1-blocking antibody that blocks the interaction between the PD-1 receptor and its ligands, PD-L1 and PD-L2. It is an IgG4 kappa immunoglobulin blocking antibody. By blocking the PD-1 receptor pathway, the drug may block the PD-1-mediated negative regulatory T-cell signaling in order to allow for T-cell activation to create an antitumor immune response. It is likely that the function of pembrolizumab is much more complex, involving an intricate relationship between PD-1 and other immune modulatory pathways. Notably, PD-1 acts via a distinct mechanism from CTLA-4 to inhibit T-cell activation.Citation13
Pembrolizumab has a long elimination half-life of 26 days, with steady-state concentrations reached by 18 weeks on an every 3-week regimen with dose-proportional pharmacokinetic parameters in the dose range of 2–10 mg/kg every 3 weeks. No clinically important differences in pembrolizumab clearance were noted between patients with renal impairment and patients with normal renal function, nor were there differences in clearance between patients with mild hepatic dysfunction and normal function.Citation14
Efficacy, safety, and tolerability of pembrolizumab
Melanoma
Patients with advanced melanoma were initially enrolled in a Phase I clinical trial of pembrolizumab for patients with advanced solid tumors (KEYNOTE-001, NCT01295827) in late 2011. When initial efficacy data were first reported in abstract form in 2012 in patients with melanoma, the study protocol had been amended to add additional cohorts of melanoma patients.Citation15 Accrual proceeded rapidly; 135 patients with advanced melanoma who either had or had not previously been treated with ipilimumab were treated in sequential nonrandomized cohorts with pembrolizumab at doses of 10 mg/kg every 2 weeks, 10 mg/kg every 3 weeks, and 2 mg/kg every 3 weeks. Also, 276 additional patients were subsequently enrolled in two randomized cohorts, and 173 of these patients had disease defined as ipilimumab refractory and the remaining 103 patients were ipilimumab naïve. Within each subset, patients were randomized to receive pembrolizumab at a dose of either 10 or 2 mg/kg every 3 weeks.
Data from the first 135 patients enrolled in KEYNOTE-001 were published in 2013, with a centrally confirmed RECIST objective response rate of 38% and no difference in the response rates of those patients who had and had not received prior ipilimumab. Responses were very durable, with 42 of the 52 responding patients (81%) still receiving treatment at the time of analysis.Citation16
Data from the 173 ipilimumab-refractory patients enrolled in the randomized cohorts of KEYNOTE-001 were published in 2014, with no difference in response rates for patients randomized to receive pembrolizumab 2 mg/kg every 3 weeks or 10 mg/kg every 3 weeks.Citation17 The centrally confirmed objective response rate was 26% in both the dose cohorts of ipilimumab-refractory patients. The lack of dose dependence of response rate was also confirmed in ipilimumab treatment-naïve patients. The centrally confirmed response rates for the 103 ipilimumab-naïve patients randomized to receive pembrolizumab at doses of either 2 mg/kg every 3 weeks or 10 mg/kg every 3 weeks were 33% and 40%, respectively (P=0.4835), which were not statistically different.Citation18
Overall, the objective response rate from all 411 patients with advanced melanoma enrolled in all cohorts of KEYNOTE-001 was 34%, with some amount of tumor shrinkage in 72% of patients. Responses were observed in patients with ECOG performance status 0 or 1, LDH level elevated or normal, and with or without brain metastases. Responses were observed in all subsets of patients, regardless of their M stage, BRAF mutation status, prior BRAF/MEK inhibitor treatment, prior ipilimumab, or prior chemotherapy.Citation19 There was evidence that patients with a smaller baseline tumor size had a significantly higher response rate and survival compared to patients with a larger baseline tumor size, as defined by the sum of the long dimension of RECIST target lesions at baseline. Baseline tumor size was the only factor predictive of response and prognostic of survival in a multivariate analysis including M stage and prior treatment, among other factors.Citation20
Patients with increased PD-L1 expression were more likely to have an objective response compared to patients with no PD-L1 tumor expression, with response rates of 49% and 13%, respectively (P=0.0007). PD-L1 positivity was defined as staining in 1% or more of tumor cells.Citation19
Of note, overall response rates (ORRs) were slightly higher when assessed by investigator review using the immune-related response criteriaCitation21 compared to the RECIST response rates reported by the independent central radiology review (37% vs 34%, respectively).Citation22 Patients with progression by RECIST but nonprogression by immune-related response criteria had an improved overall survival compared to patients with progressive disease by both criteria. In general, patients with mild asymptomatic radiographic progression and without any unacceptable toxicity can continue treatment with pembrolizumab until repeat radiographic confirmation of progressive disease; in some cases, either a delayed response or a response after initial mild progression or the appearance of new lesions can be observed.
More recently, pembrolizumab has been compared to cytotoxic chemotherapy in a randomized trial (KEYNOTE-002, NCT01704287) in patients with advanced melanoma who had previously been treated with ipilimumab and also a BRAF or MEK inhibitor if BRAF-mutant melanoma. Objective responses were observed in 21% and 25% of patients treated with pembrolizumab 2 and 10 mg/kg every 3 weeks, respectively. There was no statistical difference in the response rates for the two doses; both response rates were higher than that for chemotherapy (4%) as expected (P<0.0001), with durable ongoing responses in almost all patients.Citation23 Pembrolizumab resulted in significantly smaller changes from baseline compared to chemotherapy in a study of health-related quality of life in KEYNOTE-002 patients, using the global health status/quality-of-life scale of the EORTC QLQ-C30 (P=0.01).
Pembrolizumab is generally well tolerated, with serious treatment-related adverse events being rare. There were no treatment-related deaths in the 411 patients with advanced melanoma enrolled in KEYNOTE-001 or in the 540 patients enrolled in KEYNOTE-002. Treatment-related adverse events led to discontinuation in 4% of patients in KEYNOTE-001, with 12% of patients experiencing grade 3–4 treatment-related adverse events. No specific grade 3 or 4 adverse event occurred in more than 2% of patients, with 2% of patients experiencing grade 3 or 4 fatigue and less than 1% of patients experiencing grade 3 or 4 pruritus, rash, diarrhea, nausea, headache, hypothyroidism, decreased appetite, dyspnea, and increased ALT. About 8% of patients did experience hypothyroidism of any grade.
Pembrolizumab gained regulatory approval by the US Food and Drug Association (FDA) in 2014 for the treatment of patients with unresectable stage III or stage IV melanoma with disease progression after ipilimumab and, if BRAF V600-mutant melanoma, a BRAF inhibitor. A randomized Phase III trial of pembrolizumab compared to ipilimumab in previously untreated patients with advanced melanoma has completed accrual (KEYNOTE-006, NCT01866319). Additional trials of pembrolizumab in melanoma are focused on combination therapy approaches with other immune therapies or targeted therapy.
In addition to the data seen with pembrolizumab, nivolumab, the fully human IgG4 antibody targeted against PD-1, has also demonstrated clinical activity in melanoma. An updated analysis of 107 melanoma patients treated with nivolumab demonstrated an ORR of 31%, with a median response duration of 2 years.Citation24 Additionally, the combination of nivolumab with other checkpoint inhibitors, including the CTLA-4 antibody, ipilimumab, has also demonstrated promise. In results reported from a Phase I dose escalation study, ORRs appear to be higher with the combination, albeit with some increase in toxicity.Citation25
Pembrolizumab in other tumor types
While clinical data to support the efficacy of pembrolizumab have been clearly demonstrated in advanced melanoma, evidence of clinical activity has also been seen in other tumor types, highlighting the potential for the broad application of immuno-oncology across multiple diseases, even those previously not thought to be responsive to immunologic approaches ().
Table 1 Selected PD-1-based trials in advanced solid tumors
Non-small cell lung cancer
Traditionally, the mainstay of treatment for advanced non-small cell lung cancer (NSCLC) has been with platinum-based chemotherapy, though an increased understanding of the molecular pathogenesis of the disease has led to multiple novel targets for specific patient subsets in recent years.Citation26–Citation30 However, despite the potential for marked responses, for the majority of patients with advanced disease, durable responses remain rare. Historically, immunotherapeutic approaches with vaccines, IL-2, and interferon had shown limited or no benefit in the NSCLC population, and had substantial toxicity.Citation31,Citation32 However, early signals of clinical activity with immune checkpoint blockade were seen in NSCLC patient cohorts with the anti-CTLA-4 antibody ipilimumab as well as with PD-1- and PD-L1-based approaches, suggesting that lung cancers could respond to immunotherapy.Citation33–Citation35
PD-L1 expression has been studied in a number of resected NSCLC specimens, and has been seen in approximately 25%–50% of cases, underscoring a potential role for PD-1-based therapy.Citation36–Citation39 In rarer subsets, such as sarcomatoid NSCLC, more than two thirds of tumors express PD-L1.Citation40 However, the definitive prognostic significance of PD-L1 expression is yet to be determined, with mixed results across reported studies.Citation37,Citation38,Citation41,Citation42
Nivolumab was one of the first PD-1 inhibitors to demonstrate promising results in NSCLC.Citation43 In a cohort of 129 NSCLC patients treated on a Phase I trial, the ORR was 17%, and the median duration of response was 74 weeks. Consistent with prior response patterns to immunotherapy, a subset of patients appeared to derive long-term benefits, with a 1-year survival rate of 42% and a 2-year rate of 24%. These results were especially promising in light of the fact that these patients were heavily pretreated, with more than half having received at least three lines of therapy.Citation43 Results of an early-phase trial of pembrolizumab also demonstrate comparable clinical activity in previously treated patients with NSCLC.Citation44 In this study, patients were randomized to receive pembrolizumab at 10 mg/kg given either every 2 or 3 weeks. A total of 305 patients were eligible based on any detectable level of PD-L1 expression out of 450 patients screened, though some patients whose tumors were negative for PD-L1 were treated in the every 2-week cohort. The (confirmed and unconfirmed) ORR by RECIST 1.1 for patients whose tumors expressed PD-L1 was 26% (CI =15–40) in the 2-week cohort and 21% (CI =13–30) in the 3-week cohort. The majority of responses appeared to be durable. Some responses were still seen in patients whose tumors did not express PD-L1, albeit at lower rates (9%, CI =2–23). Drug-related toxicities were manageable and consistent with agents in this class, the most common being fatigue, decreased appetite, arthralgia, pruritus, and rash. Of note, four cases of drug-related grade 3/4 pneumonitis were seen. Additional patients are currently being enrolled to this study, as well as to a combination study of pembrolizumab with docetaxel for patients with NSCLC who have received at least one prior therapy (NCT01905657). In the first-line setting, pembrolizumab has also shown promising clinical activity in early-phase studies.Citation45 In a recent trial, pembrolizumab was administered at three different dosing schedules (2 mg/kg every 3 weeks, 10 mg/kg every 3 weeks, 10 mg/kg every 2 weeks) to 57 patients with advanced NSCLC whose tumors expressed PD-L1 by immunohistochemistry (IHC). Patients had not received any prior therapy for advanced NSCLC, though chemotherapy in the adjuvant setting was allowed if more than 1 year had elapsed. Eighty-four patients were screened, and 57 had tumors that expressed PD-L1; 45 met all eligibility criteria and subsequently enrolled in the study. The best ORR by RECIST for the total population was 26% (14–42). The response rate was 33% (4–78) in the 2 mg/kg cohort and 20% (6–44) and 31% (11–59) in the 10 mg/kg arms (every 3 weeks/every 2 weeks), respectively. Preliminary progression-free survival was 27 weeks, with 56% of patients remaining on treatment at the time of the analysis. Overall, toxicities compared favorably with other regimens and included fatigue, pruritus, diarrhea, and dyspnea. The only grade 3 adverse events were a pericardial effusion and pneumonitis. Larger, randomized studies in the first-line setting are planned or are currently underway.
Genitourinary cancers
Immunotherapeutic approaches have also been shown to be a viable option for subsets of patients with RCC, historically with high-dose IL-2 or interferon-α2B.Citation46,Citation47 As in melanoma, high-dose IL-2 was approved for the ability to induce durable complete responses (CRs) in a small proportion of patients; however, the treatment is associated with substantial toxicity. Interferon-α2B is currently considered as a first-line option in combination with bevacizumab for selected patients with clear cell histology.Citation48 Advances have identified other strategies that have improved outcomes, including antiangiogenic-based therapies as well as targeting mammalian target of rapamycin, though CRs with targeted agents are rare.Citation49
Most recently, the potential for PD-1-based therapies has reignited the interest in immunotherapeutic approaches for this disease. Preclinical data suggest that targeting the PD-1 pathway may show promise in RCC as well. In a large retrospective series of nephrectomy specimens, PD-1 was interestingly shown to be expressed by surrounding immune cells, and was present in approximately half of tumors with mononuclear infiltrates.Citation50 In this study, the presence of PD-1- positive cells was associated with more adverse prognostic features and a worse overall survival, suggesting a role for therapies directed against PD-1. Early clinical activity has also been seen with PD-1-based approaches for RCC. In an expansion cohort of 33 RCC patients treated on a Phase I study with nivolumab, objective responses were seen in 24% of patients treated with 1 mg/kg and in 31% of patients treated with 10 mg/kg; additionally, it appeared that many of these responses were durable.Citation35 Additionally, preclinical observations have suggested that antiangiogenic therapies may affect the tumor microenvironment and allow for synergy with immunotherapies.Citation51,Citation52 A number of combination strategies are currently underway, including a clinical trial assessing the safety of pembrolizumab in combination with the angiogenesis inhibitor pazopanib (NCT02014636).
In prostate cancer, analyses of prostatectomy specimens have shown that intra- and peritumoral lymphocytes express PD-1, suggesting that manipulation of this pathway could be of potential clinical interest.Citation53,Citation54 However, to date, there has been limited efficacy seen with PD-1-targeted therapy in castrate-resistant prostate cancer (CRPC), despite some early promise seen with other immunomodulatory agents such as ipilimumab, though limited data exist at this point.Citation55 In the Phase I trial of nivolumab, 17 patients with metastatic CRPC were treated, and no responses were seen; in the two patients whose tumors were tested, PD-L1 was not expressed, which may be an important predictor of response.Citation35
Head and neck squamous cell carcinoma
Head and neck squamous cell carcinoma is potentially curable in many patients, but recurrent or metastatic disease is difficult to treat, and once diagnosed, median survival is just over 1 year.Citation56,Citation57 Current therapeutic options are limited, and include standard cytotoxic chemotherapies or the EGFR-targeted antibody cetuximab.Citation58 In a recent study of patients with recurrent or metastatic head and neck cancer, pembrolizumab demonstrated early signals of promising clinical activity.Citation59 In this Phase Ib expansion study, patients whose tumors expressed PD-L1 (defined as >1% positive cells in stroma or on tumor cells by IHC) were treated with pembrolizumab at 10 mg/kg every 2 weeks. HPV-positive and HPV-negative patients were treated in separate cohorts. Nearly 80% of patients screened were positive for PD-L1 expression, and 60 patients were treated. Most patients had received at least one prior line of therapy. Overall, pembrolizumab appeared to be well tolerated, and the most common side effects were fatigue and pruritus. There were very few severe adverse events, the most common being rash. Approximately half of patients had a decrease in tumor size, with 20% of patients having a response by RECIST criteria; the majority of responses appeared to be ongoing. Interestingly, both HPV-positive and HPV-negative patients had similar response rates. In an exploratory analysis, the degree of PD-L1 expression appeared to correlate with response. An additional expansion of this study is currently underway, and Phase III studies in this patient population are planned.
Colorectal cancer
Analyses of tumor specimens have shown that a number of gastrointestinal malignancies express PD-L1, including approximately 50% of colorectal cancers (CRCs).Citation60 More recent analyses have looked at the association of PD-L1 expression in the context of the molecular genetics of CRC, as the role of PD-L1 continues to be defined. In a study of 77 clinically annotated CRC specimens, 47% of sporadic CRC cases had PD-1-positive intraepithelial lymphocytes.Citation61 When analyzing this by microsatellite instability (MSI), it was found that MSI-high (MSI-H) tumors had a significantly higher expression of PD-1-positive lymphocytes when compared to MSI-low (MSI-L) tumors (72% vs 39%, P<0.03). Additionally, MSI-H tumors appeared to have a higher rate of expression of PD-L1 when compared to MSI-L tumors (56% vs 21%, P=0.007). Other studies, however, have shown mismatch repair-deficient CRC to have a lower rate of PD-L1 positivity.Citation62
Clinically, there has been early evidence of activity of PD-1-based therapy in CRC. In a Phase I study of nivolumab that included 39 patients, one patient with metastatic CRC had a durable CR.Citation63 Interestingly, a pretreatment tumor specimen revealed membranous expression of PD-L1 on tumor-infiltrating macrophages and lymphocytes and on rare tumor cells, as well as PD-1-positive CD3+ T cells.Citation64 Studies are currently underway with nivolumab as well as pembrolizumab in this patient population. Given the potential differences in susceptibility to modulation with immune checkpoint inhibitors in MSI-H versus MSI-L tumors, a Phase II study of pembrolizumab in patients with CRC is currently enrolling patients (NCT01876511). In this study, patients with MSI-H and MSI-L metastatic CRC will receive pembrolizumab monotherapy in separate cohorts.
Hematologic malignancies
A number of other cancers have been shown to express PD-L1, suggesting that immune checkpoint blockade could be beneficial in these malignancies as well.Citation65 In hematologic malignancies, the PD-1 antibody CT-011 has shown evidence of clinical activity. Of 17 patients, one patient with non-Hodgkin’s lymphoma had a CR.Citation66 Trials of pembrolizumab in this setting are planned or are currently underway, including studies of pembrolizumab monotherapy as well as a combination study with lenalidomide and low-dose dexamethasone in patients with refractory multiple myeloma.
Conclusion
The successful development of PD-1-based therapy represents a breakthrough in the field of immuno-oncology that has the potential to impact treatment options for many different cancers. Pembrolizumab has demonstrated promising clinical activity in melanoma, and was recently FDA approved for patients whose disease has progressed on ipilimumab and a BRAF inhibitor if indicated. Regarding melanoma, first-line trials are currently underway or have recently been completed. In other malignancies, including NSCLC and RCC, PD-1- targeted agents, including pembrolizumab and nivolumab, have shown clear evidence of antitumor activity. The unique side effect profile of PD-1-directed therapy makes it a particularly attractive therapeutic strategy for many malignancies, where current standards are often associated with substantial toxicities. Overall, agents in this class, including pembrolizumab, appear to be very well tolerated, and relatively few serious adverse events have been noted. In the future, it is likely that combination strategies will play a key role, and will need to be tailored to specific malignancies. Additionally, continuous exploration of predictive and prognostic markers, as well as a better understanding of mechanisms of resistance, will allow for improved outcomes for many patients.
Disclosure
Dr Salama receives research funding from Bristol-Myers Squibb, and has served as a consultant for Roche-Genentech. Dr Gangadhar has received honoraria from Merck. The authors report no other conflicts of interest in this work.
References
- RosenbergSAYangJCTopalianSLTreatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2JAMA1994271129079138120958
- KirkwoodJMStrawdermanMHErnstoffMSSmithTJBordenECBlumRHInterferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684J Clin Oncol19961417178558223
- HodiFSO’DaySJMcDermottDFImproved survival with ipilimumab in patients with metastatic melanomaN Engl J Med2010363871172320525992
- KantoffPWHiganoCSShoreNDSipuleucel-T immunotherapy for castration-resistant prostate cancerN Engl J Med2010363541142220818862
- ZajacAJBlattmanJNMurali-KrishnaKViral immune evasion due to persistence of activated T cells without effector functionJ Exp Med199818812220522139858507
- WherryEJT cell exhaustionNat Immunol201112649249921739672
- VirginHWWherryEJAhmedRRedefining chronic viral infectionCell20091381305019596234
- BarberDLWherryEJMasopustDRestoring function in exhausted CD8 T cells during chronic viral infectionNature2006439707768268716382236
- TrautmannLJanbazianLChomontNUpregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunctionNat Med200612101198120216917489
- FranciscoLMSalinasVHBrownKEPD-L1 regulates the development, maintenance, and function of induced regulatory T cellsJ Exp Med2009206133015302920008522
- DayCLKaufmannDEKiepielaPPD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progressionNature2006443710935035416921384
- PetrovasCCasazzaJPBrenchleyJMPD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infectionJ Exp Med2006203102281229216954372
- ParryRVChemnitzJMFrauwirthKACTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanismsMol Cell Biol200525219543955316227604
- KEYTRUDA® (pembrolizumab) [package insert]Whitehouse Station, NJMerck2014Accessed November 26, 2014
- HamidOEfficacy and safety of MK-3475 in patients with advanced melanomaPresented at: The Society for Melanoma Research 2012 CongressHollywood, CANovember 8–11, 2012
- HamidORobertCDaudASafety and tumor responses with lambrolizumab (anti-PD-1) in melanomaN Engl J Med2013369213414423724846
- RobertCRibasAWolchokJDAnti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trialLancet201438499481109111725034862
- HamidORobertCRibasARandomized comparison of two doses of the anti-PD-1 monoclonal antibody MK-3475 for ipilimumab-refractory (IPI-R) and IPI-naive (IPI-N) melanoma (MEL) [abstr 3000]J Clin Oncol201432Suppl5S
- RibasAHodiFSKeffordREfficacy and safety of the anti-PD-1 monoclonal antibody MK-3475 in 411 patients (pts) with melanoma (MEL) [abstr LBA9000]J Clin Oncol201432Suppl5S
- JosephRWElassaiss-SchaapJWolchokJBaseline tumor size as an independent prognostic factor for overall survival in patients with metastatic melanoma treated with the anti-PD-1 monoclonal antibody MK-3475 [abstr 3015]J Clin Oncol201432Suppl5S
- WolchokJDHoosAO’DaySGuidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteriaClin Cancer Res200915237412742019934295
- HodiFSRibasADaudAEvaluation of immune-related response criteria (irRC) in patients (pts) with advanced melanoma (MEL) treated with the anti-PD-1 monoclonal antibody MK-3475 [abstr 3006]J Clin Oncol201432Suppl5S
- RibasAPuzanovIDummerRA randomized controlled comparison of pembrolizumab and chemotherapy in patients with ipilimumab-refractory melanomaPresented at: Society for Melanoma Research 2014 International CongressZurich, Switzerland13–16 November, 2014
- TopalianSLSznolMMcDermottDFSurvival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumabJ Clin Oncol201432101020103024590637
- WolchokJDKlugerHCallahanMKNivolumab plus ipilimumab in advanced melanomaN Engl J Med2013369212213323724867
- DouillardJYShepherdFAHirshVMolecular predictors of outcome with gefitinib and docetaxel in previously treated non-small-cell lung cancer: data from the randomized phase III INTEREST trialJ Clin Oncol201028574475220038723
- KwakELBangYJCamidgeDRAnaplastic lymphoma kinase inhibition in non-small-cell lung cancerN Engl J Med2010363181693170320979469
- MokTSWuYLThongprasertSGefitinib or carboplatin-paclitaxel in pulmonary adenocarcinomaN Engl J Med20093611094795719692680
- ShawATOuSHBangYJCrizotinib in ROS1-rearranged non-small-cell lung cancerN Engl J Med2014371211963197125264305
- ZhouCWuYLChenGErlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 studyLancet Oncol201112873574221783417
- Al-MoundhriMO’BrienMSouberbielleBEImmunotherapy in lung cancerBr J Cancer19987832822889703271
- SalgallerMLThe development of immunotherapies for non-small cell lung cancerExpert Opin Biol Ther20022326527811890866
- LynchTJBondarenkoILuftAIpilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II studyJ Clin Oncol201230172046205422547592
- BrahmerJRTykodiSSChowLQSafety and activity of anti-PD-L1 antibody in patients with advanced cancerN Engl J Med2012366262455246522658128
- TopalianSLHodiFSBrahmerJRSafety, activity, and immune correlates of anti-PD-1 antibody in cancerN Engl J Med2012366262443245422658127
- YangCYLinMWChangYLWuCTYangPCProgrammed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomesEur J Cancer20145071361136924548766
- VelchetiVSchalperKACarvajalDEProgrammed death ligand-1 expression in non-small cell lung cancerLab Invest201494110711624217091
- BolandJMKwonEDHarringtonSMTumor B7-H1 and B7-H3 expression in squamous cell carcinoma of the lungClin Lung Cancer201314215716322868219
- KonishiJYamazakiKAzumaMKinoshitaIDosaka-AkitaHNishimuraMB7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expressionClin Cancer Res200410155094510015297412
- VelchetiVRimmDLSchalperKASarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PD-L1)J Thorac Oncol20138680380523676558
- ChenYBMuCYHuangJAClinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up studyTumori201298675175523389362
- MuCYHuangJAChenYChenCZhangXGHigh expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturationMed Oncol201128368268820373055
- BrahmerJRHornLAntoniaSNivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC)overall survival and long-term safety in a phase 1 trial. Presented at: IASLC 15th World Conference on Lung CancerSydney, AustraliaOctober 22–302013
- GaronEBLeighlNBRizviNASafety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC)Presented at: The American Society of Clinical Oncology Annual MeetingChicago, IL, USAOctober 22–302013
- RizviNAGaronEBPatnaikASafety and clinical activity of MK-3475 as initial therapy in patients with advanced non-small cell lung cancer (NSCLC)Presented at: The American Society of Clinical Oncology Annual MeetingChicago, IL, USAMay 30–June 3, 2014
- FyfeGFisherRIRosenbergSASznolMParkinsonDRLouieACResults of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapyJ Clin Oncol19951336886967884429
- FisherRIRosenbergSAFyfeGLong-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinomaCancer J Sci Am20006Suppl 1S55S5710685660
- EscudierBPortaCSchmidingerMRenal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-upAnn Oncol201425Suppl 3iii49iii5625210086
- CoppinCLeLPorzsoltFWiltTTargeted therapy for advanced renal cell carcinomaCochrane Database Syst Rev20082CD00601718425931
- ThompsonRHDongHLohseCMPD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinomaClin Cancer Res20071361757176117363529
- FinkeJHRaymanPAKoJSBradleyJMGendlerSJCohenPAModification of the tumor microenvironment as a novel target of renal cell carcinoma therapeuticsCancer J201319435336423867518
- KoJSZeaAHRiniBISunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patientsClin Cancer Res20091562148215719276286
- SfanosKSBrunoTCMarisCHPhenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewingClin Cancer Res200814113254326118519750
- EbeltKBabarykaGFrankenbergerBProstate cancer lesions are surrounded by FOXP3+, PD-1+ and B7-H1+ lymphocyte clustersEur J Cancer20094591664167219318244
- SlovinSFHiganoCSHamidOIpilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II studyAnn Oncol20132471813182123535954
- Brandwein-GenslerMSmithRVPrognostic indicators in head and neck oncology including the new 7th edition of the AJCC staging systemHead Neck Pathol201041536120237990
- MellLKDignamJJSalamaJKPredictors of competing mortality in advanced head and neck cancerJ Clin Oncol2010281152019933920
- BrocksteinBEVokesEEHead and neck cancer in 2010: maximizing survival and minimizing toxicityNat Rev Clin Oncol201182727421278773
- SeiwertTBurtnessBWeissJA phase Ib study of MK-3475 in patients with human papillomavirus (HPV)-associated and non-HPV–associated head and neck (H/N) cancer [abstr 6011]J Clin Oncol201432Suppl5S
- DongHStromeSESalomaoDRTumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasionNat Med20028879380012091876
- GatalicaZSnyderCLYeatsKXiaoNHoltermanDLynchHTProgrammed death 1 (PD-1) lymphocytes and ligand (PD-L1) in colorectal cancer and their relationship to microsatellite instability status [abstr 3625]J Clin Oncol201432Suppl5S
- DroeserRAHirtCViehlCTClinical impact of programmed cell death ligand 1 expression in colorectal cancerEur J Cancer20134992233224223478000
- BrahmerJRDrakeCGWollnerIPhase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlatesJ Clin Oncol201028193167317520516446
- LipsonEJSharfmanWHDrakeCGDurable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibodyClin Cancer Res201319246246823169436
- SznolMChenLAntagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer–responseClin Cancer Res20131919554224048329
- BergerRRotem-YehudarRSlamaGPhase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignanciesClin Cancer Res200814103044305118483370
- RobertCLongGVBradyBNivolumab in previously untreated melanoma without BRAF mutationN Engl J Med2015372432033025399552
