Abstract
Merkel cell carcinoma (MCC) is a relatively uncommon, neuroendocrine, cutaneous malignancy that often exhibits clinically aggressive features and is associated with a poor prognosis. It typically presents as a painless, rapidly enlarging, dome-shaped red or purplish nodule in a sun-exposed area of the head and neck or upper extremities. Our understanding of MCC has increased dramatically over the last several years and the pathogenesis continues to be an area of active research. The etiology is likely multifactorial with immunosuppression, UV-induced skin damage, and viral factors contributing to the development of MCC. The recent discovery of Merkel cell polyomavirus has allowed for at least one aspect of disease development to be much better understood. In most cases, treatment consists of wide local excision with adjuvant radiation therapy. The role of chemotherapeutics is still being defined. The recent advancement of knowledge regarding the pathogenesis of MCC has led to an explosion research into novel therapeutic agents and strategies. This review seeks to summarize the current body of literature regarding the pathogenesis of MCC and potential targets for future therapies.
Introduction
Friedrich Sigmund Merkel first described “Tastzellen”, or touch cells, in the skin in 1875.Citation1 These cells would later become known as Merkel cells. Merkel cells are epithelial cells that form a complex with sensory neurons at the epidermal–dermal junction, but their role as sensory cells has been debated for years. Recent studies confirm that Merkel cells are mechanosensitive and required for appropriate afferent nerve fiber stimulation.Citation2
Merkel cell carcinoma (MCC) is a relatively uncommon, neuroendocrine, cutaneous malignancy that often exhibits clinically aggressive features and is associated with a poor prognosis. Toker, in 1972, described trabecular carcinoma of the skin.Citation3 Six years later, Tang and Toker suggested that trabecular carcinoma originated from Merkel cells, which ultimately gave rise to the term MCC.Citation4 MCC typically presents as a painless, rapidly enlarging, dome-shaped red or purplish nodule in a sun-exposed area of the head and neck or upper extremities.Citation5–Citation7 The acronym AEIOU is used to summarize the classic clinical characteristics of MCC (Asymptomatic, Expanding rapidly, Immune suppression, Older than 50 years of age, UV exposure on fair skin).Citation8 The clinical presentation is frequently mistaken for basal cell carcinoma, amelanotic melanoma, or other cutaneous malignancies. Risk factors include male sex, increased age, fair skin, previous malignancies, UV light exposure, and immunosuppression (specifically, HIV or organ transplantation).Citation9–Citation12 There is a tendency for early and frequent locoregional metastases and recurrence, and the majority of patients die from distant metastases.Citation6 Non-sun-exposed MCC variants have been described, and they tend to be associated with even worse survival.Citation13
The incidence of MCC is estimated to be approximately 0.3–0.6/100,000 in the US, and it appears to have been increasing over the last few decades.Citation12,Citation14–Citation16 Data from the SEER database indicate that in 1986, the incidence was 0.15 cases per 100,000 in the US. However, in 2001, the incidence was noted to be 0.44 cases per 100,000.Citation17 During this time period, the incidence was estimated to increase by 8% annually.Citation18 It is unclear whether this trend is due to an increasingly aged population or increased awareness and diagnosis of the disease.Citation14 It is noted that the introduction of CK-20 as a diagnostic tool for detecting MCC preceded the time period of increasing incidence.Citation14,Citation19 The incidence of MCC in Denmark from 1995 to 2006 appears to be slightly lower at 2.2 cases per million person years.Citation20 Secondary to its rarity, it is difficult, if not impossible, to conduct controlled trials to define optimal treatment regimens for MCC, and prospective, randomized data guiding the management of MCC are lacking.
Pathogenesis
In 2008, Feng et al identified the Merkel cell polyomavirus (MCPyV).Citation21 MCPyV is part of normal skin flora and is a nearly ubiquitous infection in adults.Citation22 Infection likely occurs during childhood via close contact though the exact mode of transmission remains unknown.Citation22,Citation23 The infection appears to be asymptomaticCitation24 and to persist throughout life as antibodies can be detected for decades after infection.Citation25 The level of anti-MCPyV antibodies correlates with the overall viral load in the skin.Citation26 While MCPyV is a common infection in healthy individuals, MCC remains relatively uncommon with an estimated three cases occurring per million people.Citation14,Citation27,Citation28
The oncogenesis of MCC was historically poorly understood; however, more recent technology has allowed viral and molecular oncogenic mechanisms to be elucidated. Our understanding of MCC has increased dramatically over the last several years and continues to be an area of active research. The etiology is likely multifactorial with immunosuppression, UV-induced skin damage, and viral factors contributing to the development of MCC. However, the recent discovery of MCPyV has allowed for at least one aspect of disease development to be much better understood.
MCPyV belongs to the human polyomavirus (HPyV) family. HPyVs share a common morphology, and all are non-enveloped, double-stranded DNA viruses. Generally, the genome of HPyVs can be divided into three functional parts: noncoding control region, early gene region, and late gene region. The noncoding control region contains the transcription start sites and promoter elements. The early gene region encodes small T antigen (ST) and large T antigen (LT). The late gene region encodes capsid proteins VP-1, VP-2, and VP-3.Citation29 Typically, HPyVs cause subclinical infection and only progress to extensive disease in those who are immunocompromised. Among the HPyVs, MCPyV is unique in its association with a cancer, MCC.Citation29 Feng et al found that MCPyV was integrated into the human genome in specimens of MCC, and it is estimated that between 66% and 80% of MCC specimens are positive for MCPyV.Citation21,Citation30 Clonal integration was found in both primary and metastatic specimens, suggesting that integration occurs prior to dissemination supporting the theory that MCPyV is involved in the onco-genesis of MCC. MCPyV encodes LT and ST;Citation21 both are independently required for tumor survival and proliferation.Citation31 LT targets pocket proteins regulating the cell-cycle transit including pRB, p107, and p130. A critical downstream result of this is activation of survivin, an important mediator for cancer cell proliferation.Citation32 Interestingly, MCPyV mutations resulting in premature truncation of LT are demonstrated as a consistent feature in MCPyV-derived MCC. The mutation prevents the inactivation of p53 tumor suppressor but maintains the interaction between LT, HSC-70, and pRB.Citation33 ST activates cap-dependent translation regulator, 4E-BP1, and appears to be the major transforming oncogene.Citation22 These oncogenic mechanisms continue to be investigated to further understanding of this deadly disease.
Currently, detection of active MCPyV infection is based primarily on polymerase chain reaction (PCR) amplification of viral DNA. However, a recent study demonstrated the use of fluorescence in situ hybridization (FISH) to determine the quality of viral presence within individual MCC cells. The study found similar rates of MCPyV positivity in MCC when comparing FISH with PCR. Using FISH, the authors detected two different intracellular patterns: punctate and diffuse. The punctate pattern correlated with an integrated MCPyV genome, while the diffuse pattern indicated an episomal presence of the genome. The detection of current or past exposure to MCPyV is based on the detection of anti-MCPyV antibodies by enzyme-linked immunosorbent assay.Citation26 Anti-VP1 antibodies are detected in many MCC patients, and anti-LT may be particularly useful for identifying MCPyV within MCC and for detecting tumor recurrence.Citation26,Citation34
Despite the improvement in detection methods for MCPyV infection, the effect of MCPyV positivity on prognosis in MCC has yet to be determined.Citation26 MCPyV-negative and MCPyV-positive MCC may be distinct entities. Recent studies suggest that MCPyV-positive variants are associated with better prognosis,Citation35 and differences in miRNA expression between MCPyV-positive and MCPyV-negative MCC have been described.Citation36 It is possible that MCPyV-negative MCC arises from UV-induced DNA damageCitation37 or that MCPyV-negative MCC is initially induced by MCPyV, but tumor cells lose or eradicate the MCPyV genome after this induction (the “hit-and-run” hypothesis).Citation38 MCC cell lines are divided into two groups (classic and variant) and further categorized based on morphology. It was hypothesized that MCPyV positivity was associated with the classic form of MCC. However, recent studies demonstrate MCPyV negativity in classic cell lines. Additionally, differing levels of MCPyV are seen in various MCC cell lines. There does not appear to be a simple relationship between cell morphology and MCPyV positivity or copy number. Interestingly, all MCPyV-positive cell lines were found to contain a premature stop codon resulting in the aforementioned truncated LT.Citation39 It appears that a sequential number of events may be required for the development of MCC. First, MCPyV integrates into the human domain. Second, expression of T antigens leads to unlicensed viral replication. Third, mutations in the viral domain result in the prevention of viral replication and virion formation conferring protection to the cancer cells from immune targeted destruction.Citation22,Citation40
The molecular mechanisms underlying viral replication and cancer development in MCC continue to be an area of ongoing research. The replication cycle of MCPyV has yet to be elucidated as it has been impossible to cultivate the virus.Citation26 The functional domains of MCPyV LT have been examined. In order for LT to function appropriately, it must be localized in the nucleus. However, a previously identified nuclear localization motif is nonessential to this localization process. Furthermore, LT interaction with HSC-70 is required for growth promotion and induction of E2F target genes.Citation41 LT reduces the expression of Toll-like receptor 9, a key receptor in the innate immune response, via downregulation of the C/EBPβ transactivator providing a mechanism by which MCPyV may subvert the innate immune system.Citation42 ST expression downregulates NF-κB-targeted transcription via interactions with the regulatory protein NEMO, cytoplasmic kinase IκB, and cellular phosphatases PP4c and PP2A Aβ. These interactions prevent the nuclear translocation of NF-κB resulting in the downregulation of a number of genes involved in the innate immune response. These findings may at least in part explain how MCC subverts the immune response, persists, and replicates within host cells.Citation43 Similar to other human tumor viruses, cell-mediated immune response appears to be critical in suppressing MCC. Lending support to the importance of cell-mediated immunity is the increased risk for MCC in HIV infection and post-transplant patients. Additionally, the increased risk seen in elderly patients may be due to age-related loss of cell-mediated immunity.Citation22
ST expression induces microtubule destabilization by affecting the phosphorylation status of stathmin, a microtubule-associated protein, via interactions with cellular phosphorylase subunits. Microtubule destabilization may result in an increasingly mobile and possibly metastatic phenotype. Consequently, chemotherapeutics stabilizing microtubules or targeting stathmin expression may offer novel therapeutic approaches to the treatment of MCC.Citation44
MCPyV has been detected by PCR in other non-melanoma skin cancers including squamous cell carcinoma, basal cell carcinoma, and Bowen disease in immunocompromised patients further confusing the picture in terms of the significance of MCPyV infection in MCC.Citation45 However, MCPyV DNA loads are typically much lower in non-MCC cutaneous neoplasms than in MCC, and MCPyV LT is not detected in non-MCC cutaneous neoplasms.Citation46 The presence of MCPyV has been sought in various other cancers including small cell lung carcinoma,Citation47 melanoma, ovary, breast, bowel,Citation48 but has not been identified. Consequently, MCPyV is only linked to tumorogenesis in MCC.Citation29 There appear to be ethnic and geographical differences in MCPyV infection. MCPyV is seen in MCC in Korean and Japanese patients.Citation49,Citation50 The Japanese strains appear to be distinct from the Caucasian strains.Citation51 Further, there may be geographically related strains of MCPyV spanning five continents.Citation52
Certainly, there remain a large number of unanswered questions regarding the pathogenesis of MCC, and the recognition of multiple variants indicates the need for further research to determine the impact of MCPyV, UV-induced skin damage, cell variants, molecular mechanisms, and immunologic microenvironment in the development, behavior, and prognosis of MCC. This understanding will drive the development of therapeutic strategies for MCC in the future.
Histopathology
The histopathologic pattern of MCC is a localized, dermal proliferation of uniform, small blue cells with scant cytoplasm and a high mitotic rate ( and ). Ultrastructurally, cells are characterized by neurosecretory (dense core) granules, cytoplasmic processes, and intermediate filaments surrounding medium-sized nuclei.Citation6,Citation15,Citation53 Approximately 80%–90% of MCC specimens are positive for cytokeratin 20 (CK20), which stains in a classic para-nuclear dot-like distribution.Citation15,Citation19,Citation53 CK20, along with other immunohistochemical markers including synaptophysin, chromogranin A, thyroid transcription factor-1 (TTF-1), HMB 45, and S100 help distinguish MCC from other tumors such as melanoma, lymphoma, and cutaneous metastases of small cell carcinoma of the lung. MCC typically stains positively for synaptophysin and chromogranin A and negatively for TTF-1.Citation18,Citation26,Citation54–Citation60
Figure 1 Hematoxylin and eosin slide 2×10, Merkel cell carcinoma.
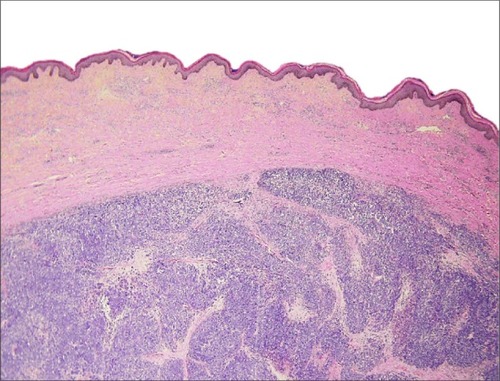
Figure 2 Hematoxylin and eosin slide 40×10, Merkel cell carcinoma.
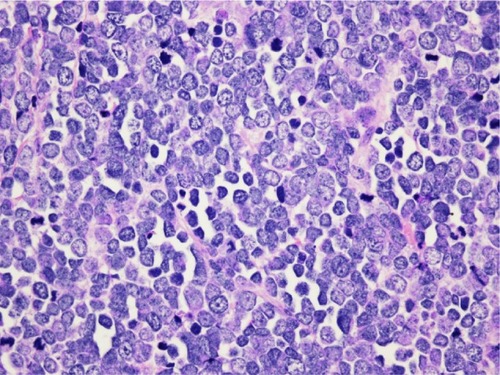
MCC is often difficult to distinguish from other neuroendocrine carcinomas. MCC has been noted to occur in the submandibular gland and to arise from nasal mucosa.Citation61,Citation62 Neuroendocrine salivary carcinomas are rare. They are divided into “Merkel cell type” and “pulmonary type”.Citation61 CK20 can aid in distinguishing between these types as CK20 positivity is a strong predictor of MCC.Citation63 However, cases of primary submandibular MCC negative for CK20 are reported.Citation61 Small cell carcinoma of the parotid is a rare diagnosis that warrants mention as it is extremely difficult to differentiate from MCC. In fact, it may represent a metastasis from an occult or regressed cutaneous MCC. Similar to MCC, small cell carcinoma of the parotid stains positive for CK20 (in a para-nuclear dot-like pattern), neuron-specific enolase, and chromogranin A. While MCC typically stains negative for TTF-1, the staining pattern for this marker in small cell carcinoma of the parotid is less clear. MCPyV positivity was useful as a distinguishing feature, but recently a study demonstrated MCPyV positivity in small cell carcinoma of the parotid. It is unclear whether these cases represent metastasis from occult cutaneous MCC or whether MCPyV plays an oncogenic role in the parotid gland.Citation64
Prognostic factors
Several factors related to MCC are associated with poorer survival outcomes. Advanced age (.75 years), male sex, lip primary site, tumor extension beyond the dermis, increasing tumor size, and positive margins are associated with reduced survival.Citation7,Citation65 Additionally, high mitotic figure count is associated with worse survival, and the immunodetection of mitotic figures in combination with G2+ tumor nuclei with histone-associated mitotic marker H3K79me3T80ph is shown to be a significant predictor of impaired survival when compared to G2+ tumor nuclei with histone-associated mitotic marker phosphohistone H3 and manual mitotic figure count alone.Citation66
Regarding immunosuppression, studies show that worse outcomes are seen with immunosuppressive states and vitamin D deficiency.Citation67,Citation68 Behr et al recently found that the presence of CD3, CD4, CD8, CD8/CD4 ratio, CD68-positive cells, neutrophils, or the presence of PD-L1-positive immune cells within the tumor or in the tumor periphery were not associated with overall or recurrence-free survival. However, they noted that the presence of tertiary lymphoid structures within the tumor was associated with increased recurrence-free survival.Citation69 This is in contrast to a previous study that demonstrated better survival with increased number of infiltrating T-cells.Citation70
As with many head and neck malignancies, both locoregional and distant nodal metastasis are respectively and independently associated with poorer disease-specific survival.Citation7,Citation65 Other work indicates that positive sentinel lymph nodes are associated with increased local recurrence rates.Citation71,Citation72 While Smith et al also found that the presence of multiple positive nodes did not independently predict disease-specific survival when positive nodes were found,Citation7 a recent study found that the number of positive nodes was inversely correlated to the 5-year survival.Citation73 Lack of histopathologic nodal evaluation is also associated with worse survival when compared with pathologically proven negative nodes.Citation7,Citation74 This data indicates that pathologic evaluation of nodal metastasis should be considered in all cases of MCC.
Nodal evaluation
Patients with clinically positive nodal metastases in the setting of MCC should undergo fine needle aspiration for pathological confirmation, and those with confirmed disease should undergo surgical resection of the nodal basins. It is currently recommended that patients who are clinically node negative undergo a sentinel lymph node biopsy (SLNB) in addition to management of the primary lesion.Citation75 SLNB will detect nodal metastases in approximately one-third of patients who are clinically node negative, and locoregional recurrence rate is greatly increased in the event of positive SLNB.Citation76 SLNB is preferable to elective neck dissection as it has decreased postoperative morbidity and superior shoulder function.Citation77
Imaging
There is no standard imaging algorithm recommended for MCC.Citation15 Peloschek et al recommend ultrasonography as the initial imaging modality to assess for nodal metastasis, as it is cost effective.Citation78 However, computed tomography (CT) is routinely used for this purpose as well. CT is more effective in assessing nodal status than magnetic resonance imaging (MRI).Citation79 Positron emission tomography (PET)/CT has similar sensitivity and specificity as conventional imaging when assessing lymph-node involvementCitation78,Citation79 and is recommended by some as first-line imaging for MCC.Citation80 In the event of negative imaging, Enzenhofer et al recommend SLNB and neck dissection as the morbidity of neck dissection is low and the information obtained has high diagnostic and preventive value.Citation80 However, the National Comprehensive Cancer Network (NCCN) guidelines recommend SLNB alone in this circumstance. In patients with positive nodes or suspected metastases, CT, MRI, or PET/CT can be obtained as all have been shown to adequately detect MCC.Citation78,Citation81–Citation86
Enzenhofer et al suggest routine follow-up imaging to include chest radiograph and CT of the head and neck at 3 months posttreatment, chest radiograph and ultrasound at 6, 9, 15, 18, 21, and 30 months posttreatment, and chest radiograph, CT head and neck, and MRI head and neck each year after treatment.Citation80 It would be reasonable to substitute PET/CT for CT at 3 months posttreatment and each year after treatment.Citation78
Staging
In 2010, a consensus staging system for MCC was introduced by the American Joint Commission on Cancer. In summary, stage I includes those with primary tumor size ≤2 cm, while stage II includes those with primary tumor size >2 cm. Stages I and II are further classified as A or B based on pathological evaluation for nodal disease. Cases in which lymph nodes were pathologically evaluated are considered either IA or IIA, while cases in which there was no pathological evaluation are considered either IB or IIB. Positive nodal disease is considered stage III. Stage III is also further classified as A or B based on the method by which nodal disease was discovered. Those discovered on pathological examination alone are considered IIIA, while those appreciated on clinical or radiological evaluation are considered IIIB. Any distant metastasis is considered stage IV.Citation75 A number of potential prognostic factors including antibodies to MCPyV capsid proteins or T-antigen oncoproteins, the presence of lymphovascular invasion, the level of immune suppression, the presence of tumor-infiltrating lymphocytes, unknown primary, and p63 expression may be incorporated into the staging algorithm in the future.Citation87
Treatment
Multidisciplinary treatment is essential to deliver optimal care to patients with MCC.Citation75,Citation88 Treatment consists of wide local excision with or without adjuvant therapy depending on the size of the primary lesion and stage of disease. There is controversy regarding the ideal margin width, and the NCCN guidelines recommend 1–2 cm margins when feasible. Studies show no difference in recurrence-free survival based on the size of surgical margin.Citation89,Citation90 Mohs micrographic surgery has been proposed as an alternative to wide local excision.Citation91–Citation93 Among the benefits of Mohs micrographic surgery are tissue conservation and identification of tumors that would otherwise require extremely wide excision margins.Citation94 Nevertheless, surgical resection of MCC with negative margins is the preferred primary modality of therapy when possible. Positive nodal disease should be treated with neck dissection and adjuvant radiotherapy.Citation89,Citation95 In the event of pathologically confirmed negative nodes, patients at high risk for locoregional recurrence should have neck dissection and/or radiotherapy of nodal basins.Citation75
Radiation therapy can be considered for primary therapy in patients who are not surgical candidates.Citation96,Citation97 Veness and Howle reported a 5-year overall survival of 40% using radiotherapy alone (50–55 Gy) in a cohort of 41 patients.Citation98 With positive margins, definitive radiotherapy is an alternative to re-resection and results in less treatment delay.Citation99
Postsurgical adjuvant radiation is often indicated in the treatment of MCC and is shown to improve outcomes. In the only randomized, prospective trial concerning adjuvant radiation, Jouary et al found that adjuvant radiation significantly reduced the probability of regional recurrence but did not affect overall survival.Citation100 A number of other studies note either lower recurrence ratesCitation90,Citation101–Citation105 or improved survival with adjuvant radiation.Citation65,Citation101,Citation106,Citation107 A systematic review of literature found that adjuvant radiation resulted in significantly higher 3-year local control rate, decreased recurrence rate, and improved 1- and 3-year overall survival rates, and that adjuvant chemotherapy did not offer any added benefit to adjuvant radiation.Citation108 Fang et al using data from the SEER database, found similar cause-specific survival in patients with MCC <1 cm with no nodal metastases when comparing surgery alone to surgery with adjuvant radiation.Citation109 Additionally, Ellis and Davis propose that adjuvant radiotherapy be considered optional in patients with the lowest risk of locoregional recurrence (immunocompetent patients with primary tumor ≤1 cm with no adverse histologic features, clear margins, and pathologically negative nodes). The relevant studies related to radiation therapy for MCC are sum-marized in . However, more research needs to define specific prognostic factors that determine ideal candidates for withholding adjuvant radiotherapy.Citation94
Table 1 Key studies assessing recurrence and survival with adjuvant radiation therapy for Merkel cell carcinoma
The use of chemotherapy is less well defined for MCC. Chemotherapy is currently used in advanced stage MCC and as palliative therapy. There is no standard choice of chemotherapeutic agent. A variety of groups have used chemotherapy regimens based on treatments for lung small cell carcinoma as it is noted to have similar neuroendocrine properties to MCC. Often, there is initial regression, but recurrences develop within 4–15 months.Citation107,Citation110–Citation118 A recent retrospective study found that adjuvant chemoradiotherapy resulted in improved overall survival when compared with adjuvant radiotherapy in patients with positive margins, tumor size at least 3 cm, and male sex.Citation65 Other studies suggest that the effect of adjuvant chemotherapy on recurrence is unclear or that there is no significant improvement in survival when compared with adjuvant radiation therapy.Citation96,Citation119–Citation121 Eng et al retrospectively reviewed 85 cases of MCC. They found that adjuvant therapy did not affect survival, and those treated with adjuvant radiation had a similar recurrence rate as those treated with adjuvant chemoradiotherapy. They concluded that the role of adjuvant chemotherapy was unclear, though they only had a small number of patients in the chemotherapy group.Citation119 One study found that adjuvant chemotherapy was actually associated with worse survival.Citation121 Currently, the literature on chemotherapy use in MCC is inadequate to suggest routine use.Citation120–Citation122 However, palliative brachytherapy offers good palliation without affecting disease or overall survival.Citation123 The relevant studies related to chemoradiotherapy for MCC are summarized in .
Table 2 Key studies assessing recurrence rate and survival with adjuvant chemoradiotherapy for Merkel cell carcinoma
Potential therapies
With the recent discovery of MCPyV and the elucidation of molecular pathways instrumental in the development of MCC, there has been an explosion of research into new therapeutic options for the disease. A recent gene expression analysis comparing MCPyV-positive MCC, MCPyV-negative MCC, and normal Merkel cells identified a number of differences in the gene expression profile. Downregulated genes were primarily involved in immune function. One of the few distinguishing factors between MCPyV-negative and MCPyV-positive MCC was the increased expression of cell adhesion molecules seen in MCPyV-negative MCC.Citation124 No definitive conclusions could be drawn; however, the study identifies a number of genes and pathways that can be further evaluated in the search for novel treatment strategies. Both antiviral and immune-modulating therapeutic options are being explored. A mouse model demonstrates the potential benefit of vaccination for MCPyV.Citation125 MCC specimens show upregulated vascular endothelial growth factor receptor,Citation126 platelet-derived growth factor receptor (PDGFR),Citation127 and KIT,Citation128–Citation131 a tyrosine kinase receptor similar to PDGFR, which have been shown to stimulate growth in MCC in vitro.Citation132 Pazopanib, a tyrosine kinase inhibitor acting against vascular endothelial growth factor receptor and PDGFR, is being explored as a potential treatment option. Similarly, imatinib mesylate, a tyrosine kinase inhibitor targeting KIT, has been investigated. Unfortunately, a phase II clinical trial was prematurely discontinued as imatinib failed to show sufficient effects on progression-free and overall survival (only one of 23 patients showed partial response, and many patients demonstrated rapid progression).Citation128 Somatostatin receptors are also upregulated in MCC. Unfortunately, somatostatin analogs have shown poor results in terms of response or time to recurrence.Citation133–Citation135 As mentioned previously, survivin is upregulated in MCC. As a result, YM155, a survivin inhibitor, is currently being tested for efficacy in MCC and has improved survival in mice with MCC.Citation32
There have been reports of spontaneous regression of MCCCitation136,Citation137 and case reports of MCC development during tumor necrosis factor-alpha inhibitor use.Citation138,Citation139 Reconstitution of cell-mediated immunity and loss of cell-mediated immunity may be responsible for these events, respectively.Citation22 T-cell infiltration may play a role in spontaneous regression as an increased number of lymphocytes have been noted surrounding tumor nests in regressing MCC compared with non-regressing MCC.Citation140 Previously, it was considered that a biopsy might induce a T-cell response resulting in regression; however, more recent studies fail to demonstrate an increase in CD8 T-cell infiltration after biopsy.Citation141 The role of the immune system has led to research into immune-modulating drugs as potential therapeutic options for MCC. Interferon therapy has been reported but to date has been unsuccessful.Citation142,Citation143 Imiquimod has been topically applied in conjunction with radiotherapy resulting in a complete response lasting 7 months.Citation144 A potential area of interest is cytokine-induced inflammatory responses, where fusion proteins containing antibodies and cytokines bind to their corresponding antigen on tumor cells to produce an immune response.Citation145 Inhibition of T antigens is another area of potential interest, and there is currently a phase II trial under way examining the results of intratumoral injection of interleukin-12 in attempt to incite an immune response against tumor cells.Citation122
A recent study associated MCC with a number of mutations in various cancer-related genes. Among these genes are PDE4DIP, MLL3, ERCC5, AURKB, ATR, TSHR, and BCL2L2.Citation146 Further studies including larger cohorts will be required to clarify the significance of and potentially identify therapeutic options targeted toward these gene mutations.
Another area of research involves telomerase reverse transcriptase (TERT). Activation of telomerase is a key step in malignant transformation in a number of cancers. TERT, the catalytic component of telomerase, expression is an important part of the activation process. TERT expression and telomerase activation are prominent in MCC. Additionally, TERT promoter mutations are seen in MCC, occur more often in sun-exposed areas, and are more common in MCPyV-negative tumors. Increased TERT mRNA expression is associated with worse survival.Citation147 Once again, studies with larger cohorts will need to confirm these findings and determine whether novel therapeutic approaches targeting TERT expression and telomerase activity would be beneficial in treating MCC. Desch and Kunstfeld also calls for the implementation of an MCC network that will allow collection of a sufficient number of patients in an MCC registry and multicenter clinical trials to explore treatment options.Citation122
Conclusion
MCC is a relatively uncommon, neuroendocrine, cutaneous malignancy. Traditional therapy involves surgical resection with negative margins, when feasible, with or without adjuvant radiation depending on the size of the primary lesion and stage of disease. The role of chemotherapy needs to be further defined. With the discovery of MCPyV and the elucidation of molecular pathways involved in the oncogenesis of MCC, there has been an increase in research into new targeted and immunologic therapeutic options. These efforts are likely to yield improved treatment strategies for patients afflicted with MCC.
Disclosure
The authors report no conflicts of interest in this work.
References
- MerkelFTastzellen and Tastkörperchen bei den Hausthieren und beim MenschenArch Mikrosk Anat187511636
- WooSHLumpkinEAPatapoutianAMerkel cells and neurons keep in touchTrends Cell Biol2015252748125480024
- TokerCTrabecular carcinoma of the skinArch Dermatol197210511071105009611
- TangCKTokerCTrabecular carcinoma of the skin: an ultrastructural studyCancer197842523112321719609
- HitchcockCLBlandKILaneyRG3rdFranziniDHarrisBCopelandEM3rdNeuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatmentAnn Surg198820722012073277546
- KoljonenVMerkel cell carcinomaWorld J Surg Oncol20064716466578
- SmithVACampERLentschEJMerkel cell carcinoma: identification of prognostic factors unique to tumors located in the head and neck based on analysis of SEER dataLaryngoscope201212261283129022522673
- HeathMJaimesNLemosBClinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU featuresJ Am Acad Dermatol200858337538118280333
- KempfWMertzKDHofbauerGFTinguelyMSkin cancer in organ transplant recipientsPathobiology201380630230924013135
- KoljonenVKukkoHTukiainenEIncidence of Merkel cell carcinoma in renal transplant recipientsNephrol Dial Transplant200924103231323519586970
- EngelsEAFrischMGoedertJJBiggarRJMillerRWMerkel cell carcinoma and HIV infectionLancet2002359930549749811853800
- AgelliMCleggLXEpidemiology of primary Merkel cell carcinoma in the United StatesJ Am Acad Dermatol200349583284114576661
- MarcovalJFerreresJRPenínRMPérezDViñalsJMMerkel cell carcinoma: differences between sun-exposed and non-sun-exposed variants – a clinical analysis of 36 casesDermatology2014229320520925278300
- AgelliMCleggLXBeckerJCRollisonDEThe etiology and epidemiology of Merkel cell carcinomaCurr Probl Cancer2010341143720371072
- HuberGFModern management of Merkel cell carcinomaCurr Opin Otolaryngol Head Neck Surg201422210911524463300
- Albores-SaavedraJBatichKChable-MonteroFSagyNSchwartzAMHensonDEMerkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based studyJ Cutan Pathol2010371202719638070
- HodgsonNCMerkel cell carcinoma: changing incidence trendsJ Surg Oncol20058911415611998
- BeckerJCMerkel cell carcinomaAnn Oncol201021Suppl 7ii81vii85
- MollRLöweALauferJFrankeWWCytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodiesAm J Pathol199214024274471371204
- KaaeJHansenAVBiggarRJMerkel cell carcinoma: incidence, mortality, and risk of other cancersJ Natl Cancer Inst20101021179380120424236
- FengHShudaMChangYMoorePSClonal integration of a polyomavirus in human Merkel cell carcinomaScience200831958661096110018202256
- AroraRChangYMoorePSMCV and Merkel cell carcinoma: a molecular success storyCurr Opin Virol20122448949822710026
- Martel-JantinCPedergnanaVNicolJTMerkel cell polyomavirus infection occurs during early childhood and is transmitted between siblingsJ Clin Virol201358128829123829968
- TolstovYLKnauerAChenJGAsymptomatic primary Merkel cell polyomavirus infection among adultsEmerg Infect Dis20111781371138021801612
- SchowalterRMPastranaDVPumphreyKAMoyerALBuckCBMerkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skinCell Host Microbe20107650951520542254
- CoursagetPSamimiMNicolJTGardairCTouzéAHuman Merkel cell polyomavirus: virological background and clinical implicationsAPMIS2013121875576923781869
- SignoriniLBelingheriMAmbrogiFHigh frequency of Merkel cell polyomavirus DNA in the urine of kidney transplant recipients and healthy controlsJ Clin Virol201461456557025467862
- ZhangCLiuFHeZSeroprevalence of Merkel cell polyomavirus in the general rural population of Anyang, ChinaPLoS One201499e10643025184447
- DalianisTHirschHHHuman polyomaviruses in disease and cancerVirology20134372637223357733
- ManganaJDziunyczPKerlKDummerRCozzioAPrevalence of Merkel cell polyomavirus among Swiss Merkel cell carcinoma patientsDermatology2010221218418820689250
- ShudaMKwunHJFengHChangYMoorePSHuman Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulatorJ Clin Invest201112193623363421841310
- AroraRShudaMGuastafierroASurvivin is a therapeutic target in Merkel cell carcinomaSci Transl Med20124133133r a56
- HoubenRSchramaDBeckerJCMolecular pathogenesis of Merkel cell carcinomaExp Dermatol200918319319819400830
- PaulsonKGCarterJJJohnsonLGAntibodies to Merkel cell polyomavirus T antigen oncoproteins reflect tumor burden in Merkel cell carcinoma patientsCancer Res201070218388839720959478
- SihtoHKukkoHKoljonenVSankilaRBöhlingTJoensuuHClinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinomaJ Natl Cancer Inst20091011393894519535775
- VeijaTSahiHKoljonenVBohlingTKnuutilaSMosakhaniNmiRNA-34a underexpressed in Merkel cell polyomavirus-negative Merkel cell carcinomaVirchows Arch2015466328929525491743
- KuwamotoSRecent advances in the biology of Merkel cell carcinomaHum Pathol20114281063107721641014
- HoubenRGrimmJWillmesCWeinkamRBeckerJCSchramaDMerkel cell carcinoma and Merkel cell polyomavirus: evidence for hit-and-run oncogenesisJ Invest Dermatol2012132125425621850029
- FischerNBrandnerJFuchsFMollIGrundhoffADetection of Merkel cell polyomavirus (MCPyV) in Merkel cell carcinoma cell lines: cell morphology and growth phenotype do not reflect presence of the virusInt J Cancer201012692133214219739110
- ShudaMFengHKwunHJT antigen mutations are a human tumor-specific signature for Merkel cell polyomavirusProc Natl Acad Sci U S A200810542162721627718812503
- HoubenRAngermeyerSHaferkampSCharacterization of functional domains in the Merkel cell polyoma virus Large T antigenInt J Cancer20151365E290E30025208506
- ShahzadNShudaMGheitTThe T antigen locus of Merkel cell polyomavirus downregulates human Toll-like receptor 9 expressionJ Virol20138723130091301924067965
- GriffithsDAAbdul-SadaHKnightLMMerkel cell polyomavirus small T antigen targets the NEMO adaptor protein to disrupt inflammatory signalingJ Virol20138724138531386724109239
- KnightLMStakaityteG1WoodJJMerkel cell polyomavirus small T antigen mediates microtubule destabilization to promote cell motility and migrationJ Virol2015891354725320307
- KassemATechnauKKurzAKMerkel cell polyomavirus sequences are frequently detected in nonmelanoma skin cancer of immunosuppressed patientsInt J Cancer2009125235636119384948
- ScolaNWielandUSillingSAltmeyerPStückerMKreuterAPrevalence of human polyomaviruses in common and rare types of non-Merkel cell carcinoma skin cancerBr J Dermatol201216761315132022803598
- WetzelsCTHoefnagelJGBakkersJMDijkmanHBBlokxWAMelchersWJUltrastructural proof of polyomavirus in Merkel cell carcinoma tumour cells and its absence in small cell carcinoma of the lungPLoS One200943e495819305499
- Sastre-GarauXPeterMAvrilMFMerkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesisJ Pathol20092181485619291712
- ChunSMYunSJLeeSCWonYHLeeJBMerkel cell polyomavirus is frequently detected in Korean patients with Merkel cell carcinomaAnn Dermatol201325220320723717012
- HattoriTTakeuchiYTakenouchiTThe prevalence of Merkel cell polyomavirus in Japanese patients with Merkel cell carcinomaJ Dermatol Sci20137029910723517683
- MatsushitaMIwasakiTKuwamotoSMerkel cell polyomavirus (MCPyV) strains in Japanese Merkel cell carcinomas (MCC) are distinct from Caucasian type MCPyVs: genetic variability and phylogeny of MCPyV genomes obtained from Japanese MCPyV-infected MCCsVirus Genes201448223324224353025
- Martel-JantinCFilipponeCTortevoyePMolecular epidemiology of Merkel cell polyomavirus: evidence for geographically related variant genotypesJ Clin Microbiol20145251687169024523477
- RamahiEChoiJFullerCDEngTYMerkel cell carcinomaAm J Clin Oncol201336329930921422993
- BeckerJMauchCKortmannRDShort German guidelines: Merkel cell carcinomaJ Dtsch Dermatol Ges20086Suppl 1S15S1618801136
- KnoeppSMHookimKPlacidoJFieldsKLRohMHThe application of immunocytochemistry to cytologic direct smears of metastatic Merkel cell carcinomaDiagn Cytopathol201341872973322144126
- DanceyALRayattSSSoonCIlchshynABrownISrivastavaSMerkel cell carcinoma: a report of 34 cases and literature reviewJ Plast Reconstr Aesthet Surg200659121294129917113506
- BobosMHytiroglouPKostopoulosIKarkavelasGPapadimitriouCSImmunohistochemical distinction between Merkel cell carcinoma and small cell carcinoma of the lungAm J Dermatopathol20062829910416625069
- KontochristopoulosGJStavropoulosPGKrasagakisKGoerdtSZouboulisCCDifferentiation between Merkel cell carcinoma and malignant melanoma: an immunohistochemical studyDermatology2000201212312611053914
- AgaimyAErlenbach-WünschKKonukiewitzBISL1 expression is not restricted to pancreatic well-differentiated neuroendocrine neoplasms, but is also commonly found in well and poorly differentiated neuroendocrine neoplasms of extrapancreatic originMod Pathol2013267995100323503646
- KaoCSWarrenSIdreesMTMerkel cell carcinoma exhibiting cytoplasmic OCT4 staining: a potential new diagnostic immunohistochemical markerAm J Dermatopathol201436327427623863554
- LombardiDAccoronaRUngariMMelocchiLBellDNicolaiPPrimary Merkel cell carcinoma of the submandibular gland: when CK20 status complicates the diagnosisHead Neck Pathol20159230931425314950
- SnowSNLarsonPOHardySMerkel cell carcinoma of the skin and mucosa: report of 12 cutaneous cases with 2 cases arising from the nasal mucosaDermatol Surg200127216517011207692
- ChanJKSusterSWenigBMTsangWYChanJBLauALCytokeratin 20 immunoreactivity distinguishes Merkel cell (primary cutaneous neuroendocrine) carcinomas and salivary gland small cell carcinomas from small cell carcinomas of various sitesAm J Surg Pathol19972122262349042291
- FisherCAHarmsPWMcHughJBSmall cell carcinoma in the parotid harboring Merkel cell polyomavirusOral Surg Oral Med Oral Pathol Oral Radiol2014118670371225457888
- ChenMMRomanSASosaJAJudsonBLThe role of adjuvant therapy in the management of head and neck Merkel cell carcinoma: an analysis of 4815 patientsJAMA Otolaryngol Head Neck Surg2015141213714125474617
- HendersonSATetzlaffMTPattanaprichakulPDetection of mitotic figures and G2+ tumor nuclei with histone markers correlates with worse overall survival in patients with Merkel cell carcinomaJ Cutan Pathol2014411184685225263506
- TarantolaTIVallowLAHalyardMYPrognostic factors in Merkel cell carcinoma: analysis of 240 casesJ Am Acad Dermatol201368342543223200197
- SamimiMTouzéALaudeHVitamin D deficiency is associated with greater tumor size and poorer outcome in Merkel cell carcinoma patientsJ Eur Acad Dermatol Venereol201428329830823368852
- BehrDSPeitschWKHametnerCPrognostic value of immune cell infiltration, tertiary lymphoid structures and PD-L1 expression in Merkel cell carcinomasInt J Clin Exp Pathol20147117610762125550797
- SihtoHJoensuuHTumor-infiltrating lymphocytes and outcome in Merkel cell carcinoma, a virus-associated cancerOncoimmunology2012181420142123243614
- KouzminaMLeikolaJBöhlingTKoljonenVPositive sentinel lymph node biopsy predicts local metastases during the course of disease in Merkel cell carcinomaJ Plast Surg Hand Surg201347213914323402426
- Santamaria-BarriaJABolandGMYeapBYNardiVDias-SantagataDCusackJCJrMerkel cell carcinoma: 30-year experience from a single institutionAnn Surg Oncol20132041365137323208132
- IyerJGStorerBE2PaulsonKGRelationships among primary tumor size, number of involved nodes, and survival for 8,044 cases of Merkel cell carcinomaJ Am Acad Dermatol201470463764324521828
- LemosBDStorerBEIyerJGPathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5,823 cases as the basis of the first consensus staging systemJ Am Acad Dermatol201063575176120646783
- EdgeSBByrdDRComptonCCAJCC Cancer Staging Manual7th edNew York, NYSpringer2010
- GuptaSGWangLCPeñasPFGellenthinMLeeSJNghiemPSentinel lymph node biopsy for evaluation and treatment of patients with Merkel cell carcinoma: the Dana-Farber experience and meta-analysis of the literatureArch Dermatol2006142668569016785370
- MurerKHuberGFHaileSRStoeckliSJComparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the n0 neck in patients with oral squamous cell carcinomaHead Neck20113391260126421837694
- PeloschekPNovotnyCMueller-MangCDiagnostic imaging in Merkel cell carcinoma: lessons to learn from 16 cases with correlation of sonography, CT, MRI and PETEur J Radiol201073231732319108971
- ColganMBTarantolaTIWeaverALThe predictive value of imaging studies in evaluating regional lymph node involvement in Merkel cell carcinomaJ Am Acad Dermatol20126761250125622552001
- EnzenhoferEUblPCzernyCErovicBMImaging in patients with Merkel cell carcinomaJ Skin Cancer2013201397312323476783
- ZagerJSBrodskySBermanCGImaging of Merkel cell carcinomaCurr Probl Cancer2010341657620371075
- AndersonSEBeerKTBanicAMRI of Merkel cell carcinoma: histologic correlation and review of the literatureAJR Am J Roentgenol200518561441144816303995
- GollubMJGruenDRDershawDDMerkel cell carcinoma: CT findings in 12 patientsAJR Am J Roentgenol199616736176208751663
- TregliaGKakhkiVRGiovanellaLSadeghiRDiagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with Merkel cell carcinoma: a systematic review and meta-analysisAm J Clin Dermatol201314643744723959776
- ConcannonRLarcosGSVenessMThe impact of (18)F-FDG PET-CT scanning for staging and management of Merkel cell carcinoma: results from Westmead Hospital, Sydney, AustraliaJ Am Acad Dermatol2010621768420082888
- HawrylukEBO’ReganKNSheehyNPositron emission tomography/computed tomography imaging in Merkel cell carcinoma: a study of 270 scans in 97 patients at the Dana-Farber/Brigham and Women’s Cancer CenterJ Am Acad Dermatol201368459259923127473
- MoshiriASNghiemPMilestones in the staging, classification, and biology of Merkel cell carcinomaJ Natl Compr Canc Netw20141291255126225190694
- SchneiderSThurnherDErovicBMMerkel cell carcinoma: interdisciplinary management of a rare diseaseJ Skin Cancer2013201318934223401779
- MorandGVitalDPézierTMerkel cell carcinoma of the head and neck: a single institutional experienceJ Skin Cancer2013201332508623365756
- GillenwaterAMHesselACMorrisonWHMerkel cell carcinoma of the head and neck: effect of surgical excision and radiation on recurrence and survivalArch Otolaryngol Head Neck Surg2001127214915411177031
- O’ConnorWJRoenigkRKBrodlandDGMerkel cell carcinoma. Comparison of Mohs micrographic surgery and wide excision in eighty-six patientsDermatol Surg199723109299339357504
- GollardRWeberRKostyMPGreenwayHTMassulloVHumbersonCMerkel cell carcinoma: review of 22 cases with surgical, pathologic, and therapeutic considerationsCancer20008881842185110760761
- BoyerJDZitelliJABrodlandDGD’AngeloGLocal control of primary Merkel cell carcinoma: review of 45 cases treated with Mohs micrographic surgery with and without adjuvant radiationJ Am Acad Dermatol200247688589212451374
- EllisDLDavisRSEvidence-based management of primary and localized Merkel cell carcinoma: a reviewInt J Dermatol201352101248125823829441
- BalakrishnanVBerrySStewBSizelandABenefits of combined modality treatment of Merkel cell carcinoma of the head and neck: single institution experienceJ Laryngol Otol2013127990891623952972
- PoulsenMRischinDMerkel cell carcinoma–current therapeutic optionsExpert Opin Pharmacother20034122187219214640917
- TuskadaAFujimuraTHashimotoASuccessful local control of cutaneous Merkel cell carcinoma on the eyelid with CyberKnife radiosurgeryEur J Dermatol201323572572624063985
- VenessMHowleJRadiotherapy alone in patients with Merkel cell carcinoma: the Westmead Hospital experience of 41 patientsAustralas J Dermatol2015561192425369110
- HrubyGScolyerRAThompsonJFThe important role of radiation treatment in the management of Merkel cell carcinomaBr J Dermatol2013169597598223898924
- JouaryTLeyralCDrenoBAdjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized studyAnn Oncol20122341074108021750118
- KokoskaERKokoskaMSCollinsBTStapletonDRWadeTPEarly aggressive treatment for Merkel cell carcinoma improves outcomeAm J Surg199717466886939409598
- MeeuwissenJABourneRGKearsleyJHThe importance of postoperative radiation therapy in the treatment of Merkel cell carcinomaInt J Radiat Oncol Biol Phys19953123253317836086
- LewisKGWeinstockMAWeaverALOtleyCCAdjuvant local irradiation for Merkel cell carcinomaArch Dermatol2006142669370016785371
- JabbourJCummingRScolyerRAHrubyGThompsonJFLeeSMerkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in earlystage disease–results from a review of 82 consecutive cases diagnosed between 1992 and 2004Ann Surg Oncol20071461943195217356954
- Medina-FrancoJUristMMFiveashJHeslinMJBlandKIBeenkenSWMultimodality treatment of Merkel cell carcinoma: case series and literature review of 1,024 casesAnn Surg Oncol20018320420811314935
- MojicaPSmithDEllenhornJDAdjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skinJ Clin Oncol20072591043104717369567
- VenessMJPereraLMcCourtJMerkel cell carcinoma: improved outcome with adjuvant radiotherapyANZ J Surg200575527528115932436
- HasanSLiuLTripletJLiZMansurDThe role of postoperative radiation and chemoradiation in Merkel cell carcinoma: a systematic review of the literatureFront Oncol2013327624294591
- FangLParvathaneniUNghiemPSurgery with or without radiotherapy for Merkel cell carcinomaInt J Radiat Oncol2008721 SupplementS508
- GeorgeTKDi Sant’agnesePABennettJMChemotherapy for metastatic Merkel cell carcinomaCancer1985565103410382990663
- McAfeeWJMorrisCGMendenhallCMWerningJWMendenhallNPMendenhallWMMerkel cell carcinoma: treatment and outcomesCancer200510481761176416136596
- RedmondJ3rdPerryJSowrayPVukeljaSJDawsonNChemotherapy of disseminated Merkel-cell carcinomaAm J Clin Oncol19911443053071862761
- AzaguryMChevallierBAtlanDGraicYDayotJPThomineEVP-16, cisplatin, doxorubicin, and bleomycin in metastatic Merkel cell carcinoma. Report of a case with long-term remissionAm J Clin Oncol19931621021047680840
- VoogEBironPMartinJPBlayJYChemotherapy for patients with locally advanced or metastatic Merkel cell carcinomaCancer199985122589259510375107
- WobserMKürzingerNUgurelSBröckerEBBeckerJCTherapy of metastasized Merkel cell carcinoma with liposomal doxorubicin in combination with radiotherapyJ Dtsch Dermatol Ges20097652152519192166
- SchlaakMPodewskiTVon BartenwerfferWInduction of durable responses by oral etoposide monochemotherapy in patients with metastatic Merkel cell carcinomaEur J Dermatol201222218719122240092
- TaiPTYuEWinquistEHammondAStittLTonitaJGilchristJChemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 casesJ Clin Oncol200018122493249910856110
- PollackSVGoslenJBSmall-cell neuroepithelial tumor of skin: a Merkel-cell neoplasm?J Dermatol Surg Oncol1982821161227061751
- EngTYBoersmaMGFullerCDCavanaughSXValenzuelaFHermanTSTreatment of Merkel cell carcinomaAm J Clin Oncol200427551051515596922
- EngTYNaguibMFullerCDJonesWE3rdHermanTSTreatment of recurrent Merkel cell carcinoma: an analysis of 46 casesAm J Clin Oncol200427657658315577435
- AllenPJBowneWBJaquesDPBrennanMFBusamKCoitDGMerkel cell carcinoma: prognosis and treatment of patients from a single institutionJ Clin Oncol200523102300230915800320
- DeschLKunstfeldRMerkel cell carcinoma: chemotherapy and emerging new therapeutic optionsJ Skin Cancer2013201332715023476782
- GaribyanLCotterSEHansenJLPalliative treatment for in-transit cutaneous metastases of Merkel cell carcinoma using surface-mold computer-optimized high-dose-rate brachytherapyCancer J201319428328723867506
- MouchetNCoquartNLebonvalletNComparative transcriptional profiling of human Merkel cells and Merkel cell carcinomaExp Dermatol2014231292893025236165
- GomezBHeLTsaiYCWuTCViscidiRPHungCFCreation of a Merkel cell polyomavirus small T antigen-expressing murine tumor model and a DNA vaccine targeting small T antigenCell Biosci2013312923856459
- Fernández-FiguerasMTPuigLMusulénEExpression profiles associated with aggressive behavior in Merkel cell carcinomaMod Pathol20072019010117115023
- KarthaRVSundramUNSilent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesisMod Pathol20082129610418084259
- SamlowskiWEMoonJTuthillRJA phase II trial of imatinib mesylate in Merkel cell carcinoma (neuroendocrine carcinoma of the skin): a Southwest Oncology Group study (S0331)Am J Clin Oncol201033549549920019577
- FeinmesserMHalpernMKaganovskyEc-kit expression in primary and metastatic Merkel cell carcinomaAm J Dermatopathol200426645846215618926
- SuLDFullenDRLoweLUherovaPSchnitzerBValdezRCD117 (KIT receptor) expression in Merkel cell carcinomaAm J Dermatopathol200224428929312142606
- StrongSShaldersKCarrRSneadDRKIT receptor (CD117) expression in Merkel cell carcinomaBr J Dermatol2004150238438514996126
- KrasagakisKFragiadakiIMetaxariMKIT receptor activation by autocrine and paracrine stem cell factor stimulates growth of Merkel cell carcinoma in vitroJ Cell Physiol201122641099110920857409
- di BartolomeoMBajettaEBuzzoniRClinical efficacy of octreotide in the treatment of metastatic neuroendocrine tumors. A study by the Italian Trials in Medical Oncology GroupCancer19967724024088625251
- MeierGWaldherrCHerrmannRMaeckeHMueller-BrandJPlessMSuccessful targeted radiotherapy with 90Y-DOTATOC in a patient with Merkel cell carcinoma. A case reportOncology200466216016315138369
- FakihaMLetertrePVuillezJPLebeauJRemission of Merkel cell tumor after somatostatin analog treatmentJ Cancer Res Ther20106338238421119285
- RichettaAGManciniMTorroniATotal spontaneous regression of advanced Merkel cell carcinoma after biopsy: review and a new caseDermatol Surg200834681582218363731
- WooffJCTritesJRWalshNMBullockMJComplete spontaneous regression of metastatic Merkel cell carcinoma: a case report and review of the literatureAm J Dermatopathol201032661461720520522
- KrishnaSMKimCNMerkel cell carcinoma in a patient treated with adalimumab: case reportCutis2011872818421416774
- Linn-RaskerSPvan Albada-KuipersGADuboisSVJanssenKZweersPGMerkel cell carcinoma during treatment with TNF-alpha inhibitors: coincidence or warning?Ned Tijdschr Geneeskd201215622A446422647228
- InoueTYonedaKManabeMDemitsuTSpontaneous regression of Merkel cell carcinoma: a comparative study of TUNEL index and tumor-infiltrating lymphocytes between spontaneous regression and non-regression groupJ Dermatol Sci200024320321111084302
- KobaSPaulsonKGNagaseKDiagnostic biopsy does not commonly induce intratumoral CD8 T cell infiltration in Merkel cell carcinomaPLoS One201277e4146522859987
- Biver-DalleCUse of interferon-alpha in two patients with Merkel cell carcinoma positive for Merkel cell polyomavirusActa Oncol201150347948020825355
- WillmesCAdamCAlbMType I and II IFNs inhibit Merkel cell carcinoma via modulation of the Merkel cell polyomavirus T antigensCancer Res20127282120212822389452
- BalducciMDe BariBManfridaSD’AgostinoGRValentiniVTreatment of Merkel cell carcinoma with radiotherapy and imiquimod (Aldara): a case reportTumori201096350851120845819
- SchramaDReisfeldRABeckerJCAntibody targeted drugs as cancer therapeuticsNat Rev Drug Discov20065214715916424916
- GravesCAJonesAReynoldsJNeuroendocrine Merkel cell carcinoma is associated with mutations in key DNA repair, epigenetic and apoptosis pathways: a case-based study using targeted massively parallel sequencingNeuroendocrinology2015101211211925531179
- XieHLiuTWangNTERT promoter mutations and gene amplification: promoting TERT expression in Merkel cell carcinomaOncotarget2014520100481005725301727
