Abstract
Background
Recent studies have shown that the lymphocyte to monocyte ratio (LMR) is a useful predictive factor in various cancers. However, the prognostic value of LMR in patients with esophageal cancer has not been reported yet. The purpose of the current study was to determine the prognostic role of LMR in esophageal squamous cell carcinoma (ESCC).
Methods
Three-hundred and forty-eight patients who had undergone esophagectomy for ESCC were included. A receiver operating characteristic curve for survival prediction was plotted to verify the optimum cut-off point for LMR. Kaplan–Meier method was used to calculate the cancer-specific survival (CSS), the difference was assessed by the log-rank test. Univariate and multivariate analyses were performed to evaluate the prognostic factors.
Results
A receiver operating characteristic curve for survival prediction was plotted to verify the optimum cut-off point for LMR, which was 2.93. Patients with LMR ≤2.93 had a significantly worse 5-year CSS than patients with LMR >2.93 (21.2% versus 59.3%, P<0.001). For subgroup analysis, the predictive value of LMR was also significant in patients with T1-2 cancer (P=0.003), T3-4a (P<0.001), and patients with (P=0.044) or without (P<0.001) nodal metastasis. In addition, the predictive value of LMR was also significant stratified by absolute lymphocyte count (P<0.001) and absolute monocyte count (P<0.001). In multivariate analysis, LMR was a significant predictive factor of CSS (P=0.010).
Conclusion
LMR is still a predictive factor for long-term survival in patients with ESCC. We conclude that 2.93 may be the optimum cut-off point for LMR in predicting survival in ESCC patients.
Introduction
Squamous cell carcinoma (SCC) and adenocarcinoma (AC) are the two most common histologic types of esophageal cancer (EC).Citation1,Citation2 In People’s Republic of China, SCC accounts for more than 95% of EC, in contrast to the predominance of AC in the West.Citation3,Citation4 Although advances have occurred in comprehensive therapy, patients with EC still has a poor prognosis due to late diagnosis, rapid growth, and high recurrence rate.Citation5,Citation6 There are important biological differences between SCC and AC, therefore, a prognostic study that takes into account the predominance of SCC in People’s Republic of China is more and more important.
It is well known that systemic inflammatory response plays an important role in cancer progression.Citation7,Citation8 The systemic inflammatory response, which is usually measured by peripheral blood-based parameters, such as C-reactive protein, neutrophil, lymphocyte or platelet count, has been shown to be a predictive factor in various cancers, including ECs.Citation9,Citation10 Moreover, lymphocyte to monocyte ratio (LMR) is another inflammatory marker. Recently studies demonstrated that LMR is associated with prognosis in several cancers, such as hematological malignancy, colon cancer, and lung cancer.Citation11–Citation13 However, to the best of our knowledge, no studies regarding the predictive value of LMR in patients with EC are available. Therefore, the aim of this study was to investigate the prognostic role of LMR in patients with esophageal squamous cell carcinoma (ESCC).
Patients and methods
During the period between January 2006 and December 2008, a retrospective analysis was conducted on 348 patients with ESCC who underwent surgery at Zhejiang Cancer Hospital (Hangzhou, People’s Republic of China). The inclusion criteria were as follows: 1) patients with ESCC confirmed by histopathology with curative esophagectomy; 2) patients who had not received neoadjuvant chemotherapy and/or radiotherapy, and 3) preoperative blood test results were obtained within 1 week prior to surgery. Ethical approval was obtained from the Ethical Committees of Zhejiang Cancer Hospital.
All patients were treated with radical esophagectomy. Patients who had received preoperative neoadjuvant therapy (chemotherapy and/or radiotherapy) were excluded from our study. During that period, the role of postoperative adjuvant chemoradiotherapy was controversial, therefore, adjuvant therapy was not mandatory.
Data on preoperative blood cell counts were extracted from our medical records. All white blood cell and differential counts (including lymphocyte count and monocyte count) were taken within 1 week prior to surgery. In this study, the LMR was defined as the absolute lymphocyte count (ALC) divided by the absolute monocyte count (AMC).
Statistical analysis
Statistical analysis was conducted with SPSS 17.0 (SPSS Inc., Chicago, IL, USA). As this series described the prognosis of patients with ESCC, a cancer-specific survival (CSS) analysis would be more appropriate. The CSS was defined as the time from surgery to cancer-related death. A receiver operating characteristic (ROC) curve for CSS prediction was plotted to verify the optimum cut-off point for LMR, ALC, and AMC. Independent Student’s t-test was used to compare groups of continuous LMR. Chi-squared test was used to determine the significance of differences for dichotomous LMR. Pearson correlation analysis was used to determine the correlation of LMR, ALC, and AMC. The CSS was calculated by the Kaplan–Meier method, and the difference was assessed by the log-rank test. A univariate analysis was used to examine the association between various prognostic predictors and CSS. Possible prognostic factors associated with CSS were considered in a multivariable Cox proportional hazards regression analysis. A P-value less than 0.05 was considered to be statistically significant.
Results
The baseline characteristics are shown in . Among the 348 patients, 45 (12.9%) were women and 303 (87.1%) were men. The mean age was 59.2±7.8 years (range 38–80 years). The histograms of ALC, AMC, and LMR are shown in . The mean ALC and AMC were 1.67±0.66 (×109/L) and 0.70±0.41 (×109/L), respectively, with a mean LMR of 3.51±3.44. There were significant positive correlations between ALC and AMC (r=0.345, P<0.001, ), ALC and LMR (r=0.174, P=0.001, ). However, there were significant negative correlations between AMC and LMR (r=-0.490, P<0.001, ).
Figure 1 The histograms of the ALC (A), AMC (B), and LMR (C).
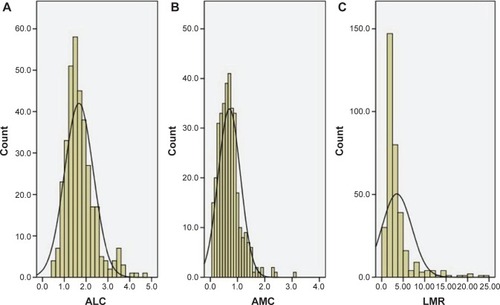
Figure 2 Positive correlations between ALC and AMC (A), ALC and LMR (B). Negative correlation between AMC and LMR (C).
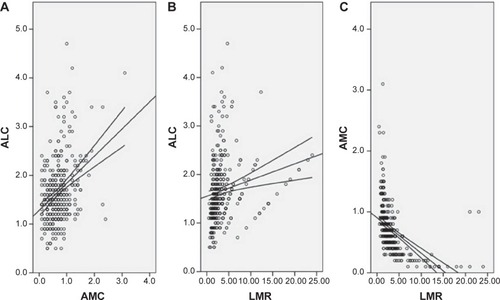
Table 1 The baseline characteristics in patients with ESCC
ROC curves for survival prediction (CSS) were plotted to verify the optimum cut-off points for ALC, AMC, and LMR, which were 1.75 (×109/L), 0.55 (×109/L), and 2.93, respectively (). Based on the cut-off value of LMR, patients were then divided into two groups for further analysis (patients with LMR ≤2.93 and patients with LMR >2.93). Overall, there were 203 (58.3%) patients with LMR ≤2.93 and 145 (41.7%) patients with LMR >2.93. Our study showed that LMR was associated with tumor length (P=0.033), depth of invasion (P=0.015), nodal metastasis (P=0.007), ALC (P<0.001), and AMC (P<0.001) ().
Figure 3 ROC curves for survival prediction (CSS) were plotted to verify the optimum cut-off points for ALC (A), AMC (B), and LMR (C).
Notes: Reference line (green); ROC line (blue).
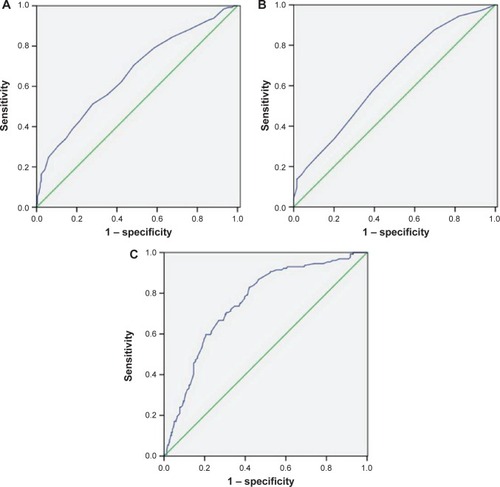
Table 2 Comparison of baseline clinical characteristics based on LMR
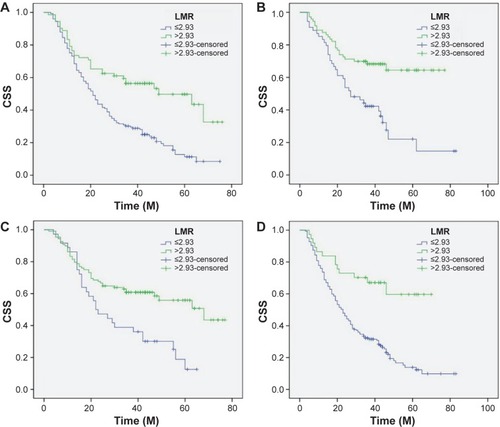
Patients with LMR ≤2.93 had a significantly worse 5-year CSS than patients with LMR >2.93 (21.2% versus 59.3%, P<0.001) (). In addition, there were also significant differences in 5-year CSS regarding ALC (52.0% versus 28.5%, P<0.001, ) and AMC (48.6% versus 28.9%, P<0.001, ). For subgroup analysis, the predictive value of LMR was significant in patients with T1-2 cancer (P=0.003), T3-4a (P<0.001), and patients with (P=0.044) or without (P<0.001) nodal metastasis (). In addition, the predictive value of LMR was also significantly stratified by ALC (P<0.001) and AMC (P<0.001) ().
Figure 4 Kaplan–Meier CSS curves stratified by LMR (A), ALC (B), and AMC (C).
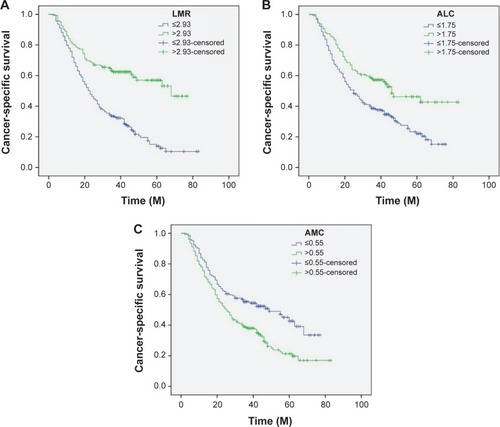
Figure 5 Kaplan–Meier CSS curves stratified by LMR in patients with T1-2 (A), T3-4a (B), and patients without (C) and with (D) nodal metastasis.
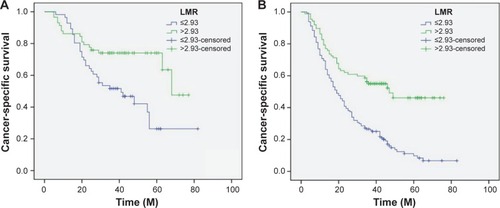
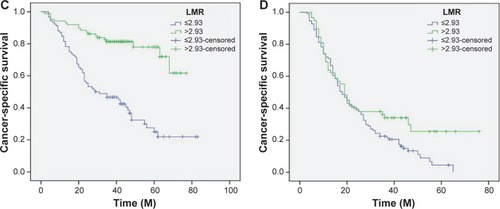
Figure 6 Kaplan–Meier CSS curves stratified by LMR in patients with ALC ≤1.75 (A), ALC >1.75 (B), AMC ≤0.55 (C), and AMC >0.55 (D).
By univariate analysis, we found that tumor length, vessel invasion, depth of invasion, nodal metastasis, ALC, AMC, and LMR had significant associations with CSS. Then multivariate Cox proportional hazards model demonstrated that LMR was an independent prognostic factor in patients with ESCC (). LMR >2.93 had a hazard ratio of 0.60 (95% confidence interval: 0.407–0.885, P=0.010) for CSS ().
Table 3 Univariate and multivariate analyses of CSS in ESCC patients
Discussion
To the best of our knowledge, this is the first study to determine the prognostic value of LMR in predicting postoperative prognosis for patients with ESCC. Our study showed that LMR is associated with tumor progression and can be considered as an independent predictive marker of prognosis in patients with ESCC. We used an ROC curve for survival prediction to verify the optimal cut-off point for LMR, and concluded the value of 2.93 may be the optimum cut-off point for LMR in predicting prognosis in ESCC patients.
In our study, we analyzed the potential prognostic role of LMR in ESCC patients who had not received neoadjuvant chemoradiotherapy mainly because chemotherapy and/or radiation will have an important impact on the systemic inflammation. Several different hematological indexes have shown prognostic significance in patients with EC.Citation14–Citation16 Recent studies have shown a strong link between cancer and inflammation. Several peripheral inflammatory cells, such as neutrophils, lymphocytes, and monocytes, were significantly associated with prognosis in various cancers.Citation17,Citation18 Hoffmann et alCitation19 demonstrated that a low lymphocyte amount might be responsible for a weak, insufficient immunologic reaction. Furthermore, Evani et alCitation20 showed that monocytes have a role in metastasis. Therefore, monocytes seem to promote tumor progression, in contrast to the role of lymphocytes.
In the present study, therefore, we initially evaluated the usefulness of LMR for predicting the postoperative CSS in patients with ESCC. Our study showed that LMR was associated with tumor length (P=0.033), depth of invasion (P=0.015), and nodal metastasis (P=0.015). This observation is in line with data from Stotz et alCitation11 but is contrary to the result of Lin et alCitation13 who suggested that LMR is not correlated with the above factors. Furthermore, controversy exists concerning the optimal cut-off points for LMR to predict survival. In our study, therefore, an ROC curve for CSS prediction was plotted to verify the optimum cut-off point for LMR, which was 2.93. Our study showed that patients with LMR ≤2.93 had a significantly worse 5-years CSS than patients with LMR >2.93. In multivariate analysis, LMR was a significant predictive factor of CSS.
It may well be that the influence of LMR on the subgroup with different T grades and N stagings is important for the understanding of its role in prognosis. In the further investigation, therefore, subgroup analysis was performed. In our study, the predictive value of LMR was also significant in patients with T1-2 cancer (P=0.003), T3-4a (P<0.001), and patients with (P=0.044) or without (P<0.001) nodal metastasis. In addition, the predictive value of LMR was also significantly stratified by ALC (P<0.001) and AMC (P<0.001). From the database of 348 patients with ESCC, our results clearly demonstrated that LMR can serve as an independent predictor of long-term survival for ESCC patients.
In conclusion, our study showed that LMR is associated with tumor progression and can be considered as an independent marker of prognosis in patients with ESCC. We conclude that 2.93 may be the optimum cut-off point for LMR in predicting survival in ESCC patients. However, larger prospective studies will need to be performed to confirm these preliminary results.
Disclosure
The authors report no conflicts of interest or financial disclosure in this work.
References
- FerlayJShinHRBrayFFormanDMathersCParkinDMEstimates of worldwide burden of cancer in 2008: GLOBOCAN 2008Int J Cancer2010127122893291721351269
- NapierKJScheererMMisraSEsophageal cancer: A review of epidemiology, pathogenesis, staging workup and treatment modalitiesWorld J Gastrointest Oncol20146511212024834141
- KeditsuKKJiwnaniSKarimundackalGPrameshCSMultimodality management of esophageal cancerIndian J Surg Oncol2013429610424426708
- LinYTotsukaYHeYEpidemiology of esophageal cancer in Japan and ChinaJ Epidemiol201323423324223629646
- FengJFZhaoQChenQXPrognostic value of subcarinal lymph node metastasis in patients with esophageal squamous cell carcinomaAsian Pac J Cancer Prev20131453183318623803101
- TachibanaMKinugasaSHiraharaNYoshimuraHLymph node classification of esophageal squamous cell carcinoma and adenocarcinomaEur J Cardiothorac Surg200834242743118502142
- MantovaniAAllavenaPSicaABalkwillFCancer-related inflammationNature2008454720343644418650914
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- FengJFZhaoQChenQXPrognostic significance of Glasgow prognostic score in patients undergoing esophagectomy for esophageal squamous cell carcinomaSaudi J Gastroenterol2014201485324496158
- FengJFChenQXSignificance of the prognostic nutritional index in patients with esophageal squamous cell carcinomaTher Clin Risk Manag2014101724379675
- StotzMPichlerMAbsengerGThe preoperative lymphocyte to monocyte ratio predicts clinical outcome in patients with stage III colon cancerBr J Cancer2014110243544024357796
- WeiXHuangFWeiYLow lymphocyte to monocyte ratio predicts unfavorable prognosis in non-germinal center type diffuse large B cell lymphomaLeuk Res201438669469824713260
- LinGNPengJWXiaoJJLiuDYXiaZJPrognostic impact of circulating monocytes and lymphocyte-to-monocyte ratio on previously untreated metastatic non-small cell lung cancer patients receiving platinum-based doubletMed Oncol20143177024927957
- VashistYKLoosJDedowJGlasgow Prognostic Score is a predictor of perioperative and long-term outcome in patients with only surgically treated esophageal cancerAnn Surg Oncol20111841130113820981494
- SatoHTsubosaYKawanoTCorrelation between the pretherapeutic neutrophil to lymphocyte ratio and the pathologic response to neoadjuvant chemotherapy in patients with advanced esophageal cancerWorld J Surg201236361762222223293
- SharaihaRZHalazunKJMirzaFElevated preoperative neutrophil:lymphocyte ratio as a predictor of postoperative disease recurrence in esophageal cancerAnn Surg Oncol201118123362336921547702
- SchmidtHBastholtLGeertsenPElevated neutrophil and monocyte counts in peripheral blood are associated with poor survival in patients with metastatic melanoma: a prognostic modelBr J Cancer200593327327816052222
- WilcoxRARistowKHabermannTMThe absolute monocyte count is associated with overall survival in patients newly diagnosed with follicular lymphomaLeuk Lymphoma201253457558022098403
- HoffmannTKDworackiGTsukihiroTSpontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importanceClin Cancer Res2002882553256212171883
- EvaniSJPrabhuRGGnanarubanVFinolEARamasubramanianAKMonocytes mediate metastatic breast tumor cell adhesion to endothelium under flowFASEB J20132783017302923616566
