Abstract
Objective
High-mobility group protein 2 (HMGA2) and epithelial–mesenchymal transition (EMT)-associated proteins play key roles in cancer progression and metastasis. However, the clinical significance of HMGA2 and its relationship with EMT markers in nasopharyngeal carcinoma (NPC) is unclear. This study aimed to assess the clinicopathological significance and prognostic value of HMGA2, E-cadherin, and vimentin in NPC.
Methods
Using immunohistochemistry, HMGA2, E-cadherin, and vimentin expression levels were evaluated in NPC (n=124) and non-tumoral inflammatory nasopharynx (n=20) tissues. The association of HMGA2 and EMT markers with clinicopathological characteristics and relationships between the protein levels and overall survival were analyzed.
Results
Compared with non-tumorous tissues, HMGA2 and vimentin levels were markedly increased in NPC tissues, whereas decreased E-cadherin levels were observed (P<0.001). Moreover, HMGA2 expression was positively correlated with vimentin levels (r=0.431, P<0.001) and negatively correlated with E-cadherin amounts (r=−0.413, P<0.001) in NPC tissues. The expression of all three proteins correlated significantly with tumor N stage, TNM stage, and 2-year metastasis. Furthermore, significant correlations were found for T stage, N stage, TNM stage, HMGA2, E-cadherin, and vimentin (all P<0.013) with poor prognosis (univariate analysis). However, multivariate analyses showed that only HMGA2 (hazard ratio [HR]: 2.683, 95% confidence interval [CI]: 1.185–6.077, P=0.018) and N stage (HR: 7.892, 95% CI: 2.731–22.807, P<0.001) were independent predictors of poor prognosis.
Conclusion
These results demonstrated that HMGA2, an independent prognostic factor, may promote NPC progression and metastasis, and is significantly associated with EMT proteins. Therefore, HMGA2 may be considered a potential therapeutic target in NPC.
Keywords:
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignancy with remarkably distinctive ethnic and geographic distributions: it is highly prevalent in Southern China and Southeast Asia.Citation1 Among head and neck cancers, most NPC cases are lowly differentiated or undifferentiated squamous cell carcinomas with a high tendency to metastasize to regional lymph nodes.Citation2,Citation3 In addition, early metastasis to the neck is common, with about 74.5% of patients presenting with regional lymph node metastasis at the time of diagnosis.Citation4 Though NPC is sensitive to radiotherapy and/or chemotherapy, treatment failure remains high due to the development of local recurrence, lymph nodes, and distant metastasis.Citation5
Epithelial–mesenchymal transition (EMT) is an important process in tumor invasion and metastasis. It is defined by the loss of epithelial morphology and acquisition of a mesenchymal phenotype.Citation6–Citation8 In the process of EMT, tumor cells escape from the primary site and invade the surrounding stroma, then enter blood or lymphatic vessels to establish new proliferating colonies. A significant hallmark of EMT is downregulation of the epithelial protein E-cadherin and upregulation of motile mesenchymal proteins such as vimentin.Citation9,Citation10 Moreover, E-cadherin or vimentin expression has been associated with metastatic dissemination and overall survival (OS) in some solid tumor types, including soft tissue leiomyosarcoma, non-small cell lung cancer, unknown primary cancers, and NPC.Citation11–Citation14
High-mobility group protein 2 (HMGA2), a non-histone nuclear-binding protein, is an important regulator of cell growth and differentiation that belongs to the HMGA protein family.Citation15 It is an oncofetal protein overexpressed in embryonic tissues and many malignant neoplasms, including lung carcinoma, breast carcinoma, ovarian carcinoma, hepatocellular carcinoma, and malignant gliomas.Citation15–Citation19 In several solid cancers, the expression levels of HMGA2 were shown to be positively correlated with tumor progression, metastasis, and poor prognosis.Citation15,Citation20–Citation22 However, studies assessing HMGA2 in NPC patients are scarce.
Although HMGA2 has also been found to play a critical role in EMT, inducing epithelial cancer invasion and metastasis,Citation23,Citation24 the interaction between expression levels of HMGA2 and EMT markers in NPC remains unclear. Therefore, this study aimed to assess the expression of HMGA2 and EMT-related markers in NPC tissues and analyze the association of HMGA2, E-cadherin, and vimentin with clinicopathological factors and patient OS.
Patients and methods
Patients and specimens
Paraffin-embedded biopsies of 124 primary NPC tissues and 20 non-tumoral inflammatory nasopharynx tissues were obtained retrospectively from the Affiliated Jiangsu Cancer Hospital, Nanjing Medical University between May 2006 and May 2011. Inclusion criteria were: 1) no radiotherapy or chemotherapy before biopsy; 2) histopathological diagnosis of NPC; 3) no distant metastasis; and 4) availability of original medical records data and complete follow-up data.
The subjects comprised 90 males and 34 females, with ages ranging from 18–74 years (median: 48 years). According to the TNM classification of Union for International Cancer Control (UICC, 2010), 44 and 80 patients presented with I–II and III–IVa–b disease stages, respectively. Among the 124 cases, 23 had lymph node or distant metastasis by imaging evaluation within 2 years after treatment. The follow-up ended in May 2014, with a median follow-up time of 50.5 months (range: 5–93 months).
This study was approved by the Ethics Committee of Jiangsu Cancer Hospital, the People’s Republic of China.
Immunohistochemistry
To assess the expression of HMGA2, E-cadherin, and vimentin, immunohistochemistry was performed according to the standard streptavidin peroxidase technique. Paraffin-embedded tissues were sectioned at 4 μm thickness, dewaxed, and rehydrated by routine techniques. All slides were subjected to the heat-induced antigen retrieval method using Tris (0.01 mmol/L, pH =6.0) buffer in a pressure cooker. Then, slides were placed in 3% hydrogen peroxide for 10 minutes to quench endogenous peroxidase. After rinsing thoroughly with phosphate-buffered saline (PBS) three times for 1 minute each, sections were further incubated with anti-HMGA2 rabbit polyclonal antibody (1:60; Abcam Ltd., Cambridge, UK), anti-E-cadherin mouse monoclonal antibody (1:120; Fuzhou Maixin Biotech, Inc., Fuzhou, People’s Republic of China), and anti-vimentin mouse monoclonal antibody (1:100; Fuzhou Maixin Biotech, Inc., Fuzhou, People’s Republic of China), respectively, overnight at 4°C. After washing in PBS, the sections were incubated with biotinylated secondary antibodies (Dako Denmark A/S, Glostrup, Denmark) for 30 minutes at room temperature, and stained with freshly prepared 3,3′-diaminobenzidine and light hematoxylin as counterstain. Known positive controls were included in every staining procedure. PBS was used to replace primary antibodies in the negative control.
Immunohistochemical evaluation
Tumor and normal tissues were histologically confirmed by hematoxylin and eosin staining. The diagnosis and immunostaining results were confirmed independently by two experienced pathologists who were blinded to the clinical data. In each case, four representative areas were selected and at least 400 tumor cells were observed at 400× magnification. The percentage of positive cells was evaluated according to the number of positive cells divided by all cancer cells under a microscope for four selected foci. The following proportion scale was adopted: 0, no positive tumor cells; 1, 1%–10% positive tumor cells; 2, 11%–50%; 3, 51%–100%. The stating intensity was interpreted by the presence of yellow- or brown-colored end product at the target antigen site. Intensity of staining (no staining, mild, moderate, and intense staining) was noted and graded as 0, 1, 2, or 3 points, respectively (0, no detectable signal; 1, mild staining –light yellow color; 2, moderate – yellow color; 3, intense – brown color). The final scores were obtained by multiplying the positive tumor grade by the tumor staining intensity score (0, 1, 2, 3, 4, 6, and 9). Final scores ≤4 and ≥6 were regarded as tumors with low and high expression, respectively.Citation13
Statistical analysis
Statistical analysis was performed using SAS software (v9.2; SAS Institute Inc., Cary, NC, USA). The expression levels of HMGA-2, E-cadherin, and vimentin and the relationships between these markers and clinicopathological parameters were analyzed using the chi-square test. The correlations between HMGA-2, E-cadherin, and vimentin were assessed by Spearman’s rank test. For survival data, Kaplan–Meier curves were generated, and statistical analysis was carried out using the log-rank test. OS was defined as the time elapsed between the beginning of radiotherapy and death from any cause or the last follow-up date. Cox regression was used for univariate analysis. Variables with prognostic value (P<0.05) in univariate analysis were selected in the final multivariable Cox proportional hazards model, while variables significantly associated with others were excluded from the final multivariable Cox proportional hazards model. P<0.05 was considered statistically significant, and all tests were two-sided.
Results
Protein expression of HMGA2, E-cadherin, and vimentin in NPC and non-tumoral samples
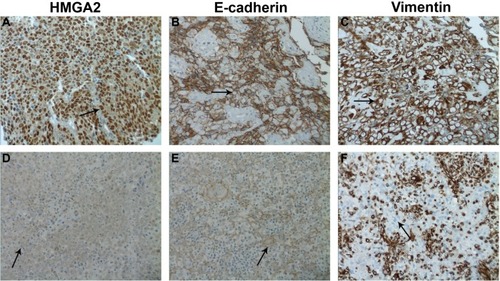
Immunohistochemical staining of HMGA2, E-cadherin, and vimentin was performed on 124 NPC samples and 20 non-tumoral inflammatory nasopharynx tissues. HMGA2 immunoreactivity was predominantly detected in the nuclei, although a weak cytoplasmic staining was observed. Among the 20 non-tumoral inflammatory nasopharynx tissues, no HMGA2 was detected; of the 124 NPC cases, 54 (43.55%) exhibited high HMGA2 expression (), indicating significant differences between the two groups (χ2=16.121, P<0.001). Diffuse membrane staining for E-cadherin was detected in cancer cells () and non-tumoral inflammatory nasopharynx tissues. High E-cadherin expression was obtained in 54/124 (43.55%) and 15/20 (75.00%) of NPC samples () and non-tumoral inflammatory nasopharynx tissues, respectively (χ2=6.826, P<0.009). Vimentin was mainly localized in the membranes and/or cytoplasm (). High vimentin expression was observed in 78/124 (62.90%) NPC specimens (), whereas no non-tumoral inflammatory nasopharynx tissues (0/20) expressed this protein (χ2=27.449, P<0.0001). See supplementary material (Figure S1) for the corresponding HE staining of .
Figure 1 Immunohistochemistry staining for HMGA2, E-cadherin, and vimentin in human nasopharyngeal carcinoma specimens.
Abbreviations: HMGA2, high-mobility group protein 2; NPC, nasopharyngeal carcinoma.
Correlation of HMGA2, E-cadherin, and vimentin expression with clinicopathological features
The clinicopathological characteristics of the patients are summarized in . Immunohistochemical analyses of NPC tissues showed that HMGA2 protein expression correlated significantly with tumor N stage, TNM stage, and 2-year metastasis status (P=0.008, 0.026, and <0.001, respectively); meanwhile, no significant correlation was observed with patient sex, age, histologic type, and T stage (P<0.05). Similarly, E-cadherin and vimentin expression levels were closely correlated with N stage, TNM stage, and 2-year metastasis status (). To assess the association between the expression levels of HMGA2, E-cadherin, and vimentin, Spearman’s correlation analysis was used. Interestingly, high vimentin expression levels were correlated with decreased E-cadherin expression (r=−0.605, P<0.001) in NPC tissues. Furthermore, HMGA2 expression was positively correlated with vimentin levels (r=0.431, P<0.001) and negatively correlated with E-cadherin amounts (r=−0.413, P<0.001) in NPC.
Table 1 Relationship between clinicopathological features and immunostaining results
Univariate and multivariate analyses of prognostic factors
Kaplan–Meier analysis was used to determine the prognostic value of HMGA2 and EMT-related proteins. Of the 124 NPC patients, a cumulative 3-year survival rate of 78.8% (95% confidence interval [CI]: 0.708–0.852) was obtained. Interestingly, the cumulative 3-year survival rate was 90.8% (95% CI: 0.806–0.957) in the low HMGA2 group, and only 66.1% (95% CI: 0.525–0.766) in the high-expression group (). In addition, a cumulative 3-year survival rate of 89.1% (95% CI: 0.757–0.953) was obtained in the low vimentin group, whereas only 71.8% (95% CI: 0.604–0.804) of patients survived in the high-expression group (). In the high E-cadherin expression group, a cumulative 3-year survival rate of 88.8% (95% CI: 0.768–0.948) was obtained, with only 70.0% (95% CI: 0.578–0.792) in the low-expression group (). A univariate analysis showed significant correlations for T stage (P=0.01), N stage (P<0.001), TNM stage (P=0.003), HMGA2 (P=0.002), E-cadherin (P=0.014), and vimentin (P=0.013) with a poor survival prognosis. In multivariate analysis of significant factors, only N stage (hazard ratio [HR]: 7.892, 95% CI: 2.731–22.807, P<0.001) and HMGA-2 (HR: 2.683, 95% CI: 1.185–6.077, P=0.018) were independently associated with OS ().
Figure 2 (A) Kaplan–Meier survival curves of overall survival in 124 patients with NPC and overall survival curves based on (B) HMGA2, (C) vimentin, and (D) E-cadherin expression levels.
Abbreviations: HMGA2, high-mobility group protein 2; NPC, nasopharyngeal carcinoma.
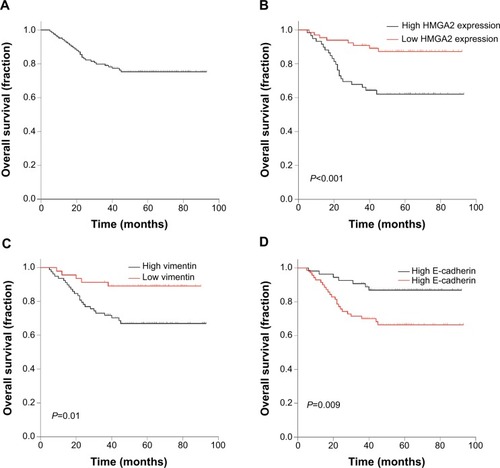
Table 2 Univariate and multivariate Cox regression analysis of overall survival in NPC patients
Discussion
In the present study, we demonstrated that HMGA2 and EMT-related markers are differentially expressed between NPC and non-tumoral tissues. Additionally, we found in NPC tissues, for the first time, a significant association between protein expression of HMGA2 and EMT indicators, and these proteins were closely linked with cancer progression and metastasis. Moreover, our results indicated that HMGA2 expression is an independent prognostic indicator for OS in NPC.
Previous studies have revealed that HMGA2 is expressed mainly in the early embryo and suppressed in human adult tissues. In addition, elevated expression of HMGA2 has been observed in a variety of cancers.Citation23,Citation25 In agreement with these reports, we found that HMGA2 expression is higher in NPC compared with non-tumorous tissues. The differential expression of HMGA2 and EMT-related proteins between non-tumorous and NPC tissues suggest a potential role for HMGA2 and EMT-related proteins in NPC carcinogenesis. These findings are consistent with previous studies described in other carcinomas and non-tumorous tissues or tumor cell lines.Citation26,Citation27
It is generally accepted that reduced E-cadherin expression coupled with increased vimentin expression is characteristic of EMT.Citation7,Citation28 Recent studies have demonstrated that ectopic expression of HMGA2 in epithelial cells induces EMT, which has been implicated in the acquisition of metastatic properties in tumor cells.Citation29–Citation33 For instance, Zha et al showed that in gastric cancer, HMGA2 overexpression induces protein changes consistent with EMT and enhanced epithelial cell invasion and migration both in vitro and in vivo.Citation30 Similarly, Wu et al found that HMGA2 silencing in ovarian cancer cell lines partially suppressed the aggressiveness of tumor cells, and caused changes in the expression of several EMT-associated genes, including vimentin and E-cadherin.Citation31 In NPC specimens, for the first time, the present results demonstrated that high HMGA2 expression is significantly and positively associated with vimentin amounts and negatively with E-cadherin levels. This finding corroborated with previous evidence supporting the potential role of HMGA2 in EMT, which is associated with vimentin expression and E-cadherin absence in NPC patients. In addition, the previous study has demonstrated that expressions of HMGA2, E-cadherin, and vimentin are correlated with T stage and bladder cancer recurrence.Citation29 A relevant finding in the present study is that HMGA2, E-cadherin, and vimentin expression levels were all significantly correlated with various aggressive behaviors, including N stage, TNM stage, and 2-year metastasis status in NPC. This finding suggests that the biomarkers may have important roles in the development and metastasis of NPC. However, no association was found with T stage. These discrepancies between the present study and the previous study might have resulted from the different antibodies used, varying staining evaluation methods, and the different biological functions of HMGA2 in various carcinomas.
Finally, a significant association between HMGA2 expression and poor prognosis has been reported in various tumor types.Citation34,Citation35 In agreement with these findings, our results provide evidence that HMGA2 might be considered a valuable prognostic biomarker in NPC. Currently, high expression of cytoplasmic E-cadherin and nuclear vimentin are considered independent prognostic factors for poor survival of NPC patients.Citation13 However, we only found HMGA2 expression and N stage as independent predictors of poor prognosis by multivariate analyses. These variations may be due to the different study populations evaluated and various clinical data quality. Additionally, another report suggested that the molecular mechanism of HMGA2 possibly involves the activation of the TGFβ signaling pathway in epithelial carcinomas.Citation23 In addition, it was shown that the genes making up the HMGA2–TET1–HOXA9 pathway are coordinately regulated in breast cancer, and together encompass a prognostic signature for patient survival.Citation36 Future studies should determine the molecular mechanism by which HMGA2 induces progression and metastasis in NPC cell lines.
In summary, our results suggested that HMGA2 and EMT protein expression levels are associated with progression and metastasis of NPC. HMGA2 may promote metastasis through EMT in NPC patients and be used as a prognostic indicator. These protein markers may have significant effects on targeted therapy; however, larger prospective studies are required to further validate these findings.
Acknowledgments
The authors thank the Department of Pathology, The Affiliated Jiangsu Cancer Hospital of Nanjing Medical University for kindly providing paraffin-embedded biopsies and the members of the Research Center of Clinical Oncology, The Affiliated Jiangsu Cancer Hospital of Nanjing Medical University for their technical assistance.
Supplementary material
Figure S1 Nasopharyngeal carcinoma specimens by HE staining.
Notes: Representative HE staining images for samples in (A) nasopharyngeal squamous cell carcinoma (high expression of HMGA2), (B) nasopharyngeal squamous cell carcinoma (high expression of E-cadherin), (C) nasopharyngeal squamous cell carcinoma (high expression of vimentin), (D) nasopharyngeal squamous cell carcinoma (low expression of HMGA2), (E) nasopharyngeal squamous cell carcinoma (low expression of E-cadherin), (F) nasopharyngeal squamous cell carcinoma (low expression of vimentin). All images, 400×.
Abbreviations: HMGA2, high-mobility group protein 2; HE, hematoxylin and eosin.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalABrayFCenterMMFerlayJWardEFormanDGlobal cancer statisticsCA Cancer J Clin2011612699021296855
- WeiWIShamJSNasopharyngeal carcinomaLancet200536594762041205415950718
- HuangTChenMHWuMYWuXYCorrelation between expression of extracellular matrix metalloproteinase inducer and matrix metalloproteinase-2 and cervical lymph node metastasis of nasopharyngeal carcinomaAnn Otol Rhinol Laryngol2013122321021523577575
- HuangCJLeungSWLianSLWangCJFangFMHoYHPatterns of distant metastases in nasopharyngeal carcinomaKaohsiung J Med Sci19961242292348683644
- ErkalHSSerinMCakmakANasopharyngeal carcinomas: analysis of patient, tumor and treatment characteristics determining outcomeRadiother Oncol200161324725611730993
- GaoDVahdatLTWongSChangJCMittalVMicroenvironmental regulation of epithelial-mesenchymal transitions in cancerCancer Res201272194883488923002209
- LimJThieryJPEpithelial-mesenchymal transitions: insights from developmentDevelopment2012139193471348622949611
- TodosiAMGavrilescuMMAniteiGMFilipBScripcariuVColon cancer at the molecular level – usefulness of epithelial-mesenchymal transition analysisRev Med Chir Soc Med Nat Iasi201211641106111123700897
- SmithATeknosTNPanQEpithelial to mesenchymal transition in head and neck squamous cell carcinomaOral Oncol201349428729223182398
- LiuANZhuZHChangSJHangXSTwist expression associated with the epithelial-mesenchymal transition in gastric cancerMol Cell Biochem20123671–219520322581441
- TianWWangGYangJPanYMaYPrognostic role of E-cadherin and Vimentin expression in various subtypes of soft tissue leiomyosarcomasMed Oncol201330140123292832
- KongFFQuZQYuanHHOverexpression of FOXM1 is associated with EMT and is a predictor of poor prognosis in non-small cell lung cancerOncol Rep20143162660266824715097
- LuoWFangWLiSYaoKAberrant expression of nuclear vimentin and related epithelial-mesenchymal transition markers in nasopharyngeal carcinomaInt J Cancer201213181863187322307379
- StoyianniAGoussiaAPentheroudakisGImmunohistochemical study of the epithelial-mesenchymal transition phenotype in cancer of unknown primary: incidence, correlations and prognostic utilityAnticancer Res20123241273128122493359
- KumarMSArmenteros-MonterrosoEEastPHMGA2 functions as a competing endogenous RNA to promote lung cancer progressionNature2014505748221221724305048
- SunMGomesSChenPRKIP and HMGA2 regulate breast tumor survival and metastasis through lysyl oxidase and syndecan-2Oncogene201433273528353723975428
- WuJWeiJJHMGA2 and high-grade serous ovarian carcinomaJ Mol Med (Berl)201391101155116523686260
- LuoYLiWLiaoHHMGA2 induces epithelial-to-mesenchymal transition in human hepatocellular carcinoma cellsOncol Lett2013541353135623599793
- LiuBPangBHouXExpression of high-mobility group AT-hook protein 2 and its prognostic significance in malignant gliomasHum Pathol20144581752175824935062
- Di CelloFHillionJHristovAHMGA2 participates in transformation in human lung cancerMol Cancer Res20086574375018505920
- MahajanALiuZGellertLHMGA2: a biomarker significantly overexpressed in high-grade ovarian serous carcinomaMod Pathol201023567368120228781
- YangGLZhangLHBoJJOverexpression of HMGA2 in bladder cancer and its association with clinicopathologic features and prognosis HMGA2 as a prognostic marker of bladder cancerEur J Surg Oncol201137326527121273026
- MorishitaAZaidiMRMitoroAHMGA2 is a driver of tumor metastasisCancer Res201373144289429923722545
- ThuaultSTanEJPeinadoHCanoAHeldinCHMoustakasAHMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transitionJ Biol Chem200828348334373344618832382
- MalekABakhidzeENoskeAHMGA2 gene is a promising target for ovarian cancer silencing therapyInt J Cancer2008123234835618452175
- BaumgartECohenMSSilva NetoBIdentification and prognostic significance of an epithelial-mesenchymal transition expression profile in human bladder tumorsClin Cancer Res20071361685169417363521
- LiuLKJiangXYZhouXXWangDMSongXLJiangHBUpregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcomeMod Pathol201023221322419915524
- LeeWYShinDYKimHJKoYHKimSJeongHSPrognostic significance of epithelial-mesenchymal transition of extracapsular spread tumors in lymph node metastases of head and neck cancerAnn Surg Oncol20142161904191124566857
- DingXWangYMaXExpression of HMGA2 in bladder cancer and its association with epithelial-to-mesenchymal transitionCell Prolif201447214615124571540
- ZhaLZhangJTangWHMGA2 elicits EMT by activating the Wnt/beta-catenin pathway in gastric cancerDig Dis Sci201358372473323135750
- WuJLiuZShaoCHMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genesCancer Res201171234935921224353
- ZhuCLiJChengGmiR-154 inhibits EMT by targeting HMGA2 in prostate cancer cellsMol Cell Biochem20133791–2697523591597
- WatanabeSUedaYAkaboshiSHinoYSekitaYNakaoMHMGA2 maintains oncogenic RAS-induced epithelial-mesenchymal transition in human pancreatic cancer cellsAm J Pathol2009174385486819179606
- YamazakiHMoriTYazawaMStem cell self-renewal factors Bmi1 and HMGA2 in head and neck squamous cell carcinoma: clues for diagnosisLab Invest201393121331133824145240
- RaskinLFullenDRGiordanoTJTranscriptome profiling identifies HMGA2 as a biomarker of melanoma progression and prognosisJ Invest Dermatol2013133112585259223633021
- SunMSongCXHuangHHMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasisProc Natl Acad Sci U S A2013110249920992523716660
