Abstract
Previous studies investigating the association between adenomatous polyposis coli (APC) gene promoter methylation and colorectal cancer (CRC) have yielded conflicting results. The aim of this study was to comprehensively evaluate the potential application of the detection of APC promoter methylation to the prevention and treatment of CRC. PubMed, Embase, and MEDLINE (results updated to October 2014) were searched for relevant studies. The effect size was defined as the weighted odds ratio (OR), which was calculated using either the fixed-effects or random-effects model. Prespecified subgroup and sensitivity analyses were conducted to evaluate potential heterogeneity among the included studies. Nineteen studies comprising 2,426 participants were selected for our meta-analysis. The pooled results of nine studies comprising a total of 740 subjects indicated that APC promoter methylation was significantly associated with CRC risk (pooled OR 5.53; 95% confidence interval [CI] 3.50–8.76; P<0.01). Eleven studies with a total of 1,219 patients evaluated the association between APC promoter methylation and the presence of CRC metastasis, and the pooled OR was 0.80 (95% CI 0.44–1.46; P=0.47). A meta-analysis conducted with four studies with a total of 467 patients found no significant correlation between APC promoter methylation and the presence of colorectal adenoma (pooled OR 1.85; 95% CI 0.67–5.10; P=0.23). No significant correlation between APC promoter methylation and patients’ Dukes’ stage, TNM stage, differentiation grade, age, or sex was identified. In conclusion, APC promoter methylation was found to be significantly associated with a higher risk of developing CRC. The findings indicate that APC promoter methylation may be a potential biomarker for the carcinogenesis of CRC.
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide and has been estimated as the third most frequently diagnosed cancer in both men and women in the USA.Citation1 The overall survival rate of patients with CRC is highly dependent upon the stage of the disease at the time of diagnosis. The estimated 5-year survival rate ranges from 85% to 90% for patients with stage I CRC to <5% for patients with stage IV CRC.Citation2 Although the annual death rate has declined by 3% for CRC in the past 10 years, nearly 142,820 US residents were diagnosed with CRC in 2013.Citation1 Several risk factors, including obesity, red meat consumption, and cigarette smoking, have been found to be associated with a higher risk of developing CRC.Citation3 Additionally, genetic and epigenetic alterations play crucial roles in the pathogenesis of CRC.Citation4–Citation6
It has been demonstrated that DNA methylation at CpG islands and its associated silencing of gene expression are important causes of the tumorigenesis of CRC.Citation2,Citation7 These epigenetic changes substantially contribute to cellular transformation, particularly if these changes affect the genes that are involved in maintaining genomic stability. Due to the high sensitivity, specificity, and convenience of methylation detection methods, mounting evidence has indicated that DNA methylation patterns may be promising biomarkers when predicting one’s risk of developing CRC, as well as for determining patient outcomes.Citation8 Among the hypermethylated genes that have been identified in CRC, the adenomatous polyposis coli (APC) gene is a well-characterized tumor suppressor gene. The APC gene, which is located at chromosomal band 5q21–q22, comprises 15 exons. Its tumor-suppressing activity is mainly attributed to regulation of the intracellular level of β-catenin within the Wingless signal transduction pathway.Citation9 It has been demonstrated that APC mutations are one of the earliest events that occur in the initiation and progression of CRC.Citation10,Citation11 In addition, previous oncogenetic tree analysis has shown that APC alterations are the first to occur in an independent branch, and these predispose an individual to other gene alterations that occur in the pathways associated with colon tumorigenesis.Citation12 To date, several studies have evaluated the association between APC promoter methylation and the risk of developing CRC (or the clinical characteristics of CRC) in patients. However, the results of these studies remain a matter of debate. Compared with in silico analyses, which are also dependent upon computer-based statistical analysis methods and have been used in the prediction of disease-associated gene mutation,Citation13 the design of new drugs,Citation14 and the analysis of molecular and structural mechanisms,Citation15,Citation16 meta-analyses have been widely applied to identify potential sources of disagreement between study results. Therefore, we conducted a meta-analysis to comprehensively evaluate the association of APC promoter methylation with CRC risk and the clinical characteristics observed in CRC patients in order to provide evidence for the future application of APC in the prevention and treatment of CRC.
Methods
Search strategy
We conducted a literature search using the PubMed (search updated to October 2014; http://www.ncbi.nlm.nih.gov/pubmed/), Embase, and MEDLINE (search updated to October 2014; http://www.embase.com/) databases using the following search terms located in either the title or abstract: (“adenomatous polyposis coli” or APC) and (methylation, hypermethylation, methylated, or hypomethylation) and (“colorectal neoplasms”, “colorectal cancer”, “colon cancer”, “rectal cancer”, “colorectal carcinoma”, “rectal carcinoma”, “colon carcinoma”, “colon neoplasms”, or “rectal neoplasms”). The search was limited to human studies. Additionally, we hand-searched the references of the review articles and, as needed, contacted the first author of a given paper to obtain any missing data.
Study selection
Studies were selected for the current meta-analysis according to the following criteria: they determined APC promoter methylation in specimens of colorectal tissue, blood, plasma, serum, buffy coat, or urine; and they provided sufficient information to evaluate odds ratios (ORs) and 95% confidence intervals (CIs). In addition, when the same author or group reported results that were obtained from the same patient population in more than one article, only the most recent or most informative report was included. Our exclusion criteria were: review articles or conference reports; a lack of information about the degree of APC promoter methylation in patient cases and controls; and when screening for methylation of the APC promoter was conducted in cell lines.
Quality assessment
The assessment of study quality was conducted independently by two reviewers using the Newcastle-Ottawa Scale (NOS).Citation17 The NOS evaluation system consists of three parameters (selection, comparability, and outcome) and assigns a maximum of four points for selection, two points for comparability, and three points for outcome. A NOS score >6 indicates a higher quality study, whereas a score ≤6 indicates a lower quality study. Any discrepancies between the two reviewers were settled by a third reviewer.
Data extraction
The data were extracted independently by two authors, and discrepancies were resolved by consensus including a third author. The data were collected using a pilot-tested data extraction form that included the following items: the first author’s name; the year of publication; the number of participants that exhibited APC promoter methylation both among specific cases and controls; the screening methods used; and the demographic and clinical characteristics of the patients. All procedures conformed to the established guidelines for the meta-analysis of observational studies in epidemiology.Citation18
Statistical analysis
The meta-analysis was performed using Stata software (version 11; StataCorp LP, College Station, TX, USA). The association between APC promoter methylation and the risk of developing CRC or its clinical characteristics (such as Dukes’ stages, TNM stages, metastasis, differentiation grade, as well as differences based on patient sex and age) was measured either by weighted OR by taking into account the 95% CI. We tested for heterogeneity among the studies using the chi-square-based Q-test and the I2 statistic of inconsistency. Significant heterogeneity was defined as a chi-square test P-value <0.10 or as an I2 statistic >50%.Citation19 We used a random-effects model when significant heterogeneity was observed among the studies; otherwise, we used a fixed-effects model. For a two-tailed significance level of 5%, the probability of rejecting the null hypothesis when it was false was termed the power, which was defined as 1 – β.
Funnel plots and Egger’s test were used to assess publication bias. Additionally, prespecified subgroup analyses, which included the patients’ ethnicities, as well as the test samples, testing methods, and sample sizes, were conducted to evaluate potential sources of heterogeneity within the studies investigating the association between APC promoter methylation and the risk of CRC or CRC metastasis. Moreover, sensitivity analyses were performed to examine the influence of each study on the pooled OR by serially omitting each individual study and pooling the remaining studies.
Results
Results of the literature search
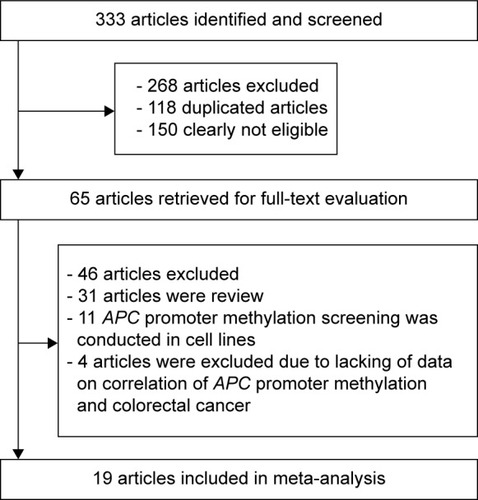
illustrates the detailed process used for study selection. In summary, a total of 333 articles were initially identified; of these, 268 articles were excluded, either based on duplicate results or because they were deemed to be irrelevant to this meta-analysis after careful review of the titles and abstracts. Among the 65 studies that remained, an additional 46 articles were excluded for various reasons: 31 were review articles; eleven were discarded because screening of APC promoter methylation was performed using cell lines; and four were excluded because of a lack of data on the association between APC promoter methylation and the development of CRC. Thus, 19 articles were ultimately selected for inclusion in the meta-analysis.Citation20–Citation38
Study characteristics
The characteristics of the studies that met all of the established inclusion criteria for the meta-analysis are presented in . Nine eligible studies presented data on the association between APC promoter methylation and the risk of developing CRC. Eleven studies assessed the correlation of APC promoter methylation with the presence of CRC metastasis. Four studies reported the association between APC promoter methylation and the presence of colorectal adenoma. In addition, the numbers of articles that reported the associations between APC promoter methylation and age of the CRC patients, sex of the CRC patients, and CRC differentiation status were 3, 6, and 4, respectively. Of the 19 included studies, ten enrolled Asian participants and nine enrolled Caucasian participants. For the detection of APC promoter methylation, 12 studies used methylation-specific polymerase chain reaction, five used quantitative real-time methylation-specific polymerase chain reaction (Q-MSP), and two used the pyrosequencing method. In addition, 17 studies used colorectal tissue specimens to screen for APC promoter methylation, while two studies used plasma or serum samples.
Table 1 Characteristics of the studies included in the meta-analysis
Data quality
We estimated the quality of the studies using the NOS evaluation system, and the results showed that 15 of the 19 studies were classified as high-quality (NOS score >6) and the remaining four trials were classified as lower-quality. The mean NOS score of the studies was 7. Most of the studies did not use community controls when we conducted an assessment of comparability.
Methylation of the APC promoter and CRC
As shown in , the pooled outcome of nine studies comprising 740 subjects indicated that APC promoter methylation was significantly associated with an increased risk of developing CRC (pooled OR 5.53; 95% CI 3.50–8.76, P<0.01; power 1.0). Eleven studies comprising 1,219 patients evaluated the association between APC promoter methylation and CRC metastasis, and the pooled OR was 0.80 at a 5% significance level with 5% power (95% CI 0.44–1.46, P=0.47; ). Four studies assessed the association between APC promoter methylation and the presence of colorectal adenoma, and the pooled OR was 1.85 at a 5% significance level with 94% power (95% CI 0.67–5.10, P=0.23; ). We also evaluated the association between APC promoter methylation and patient age, patient sex, TNM stage, Dukes’ stage, and differentiation grade, and no significant association was identified between APC promoter methylation and these specific parameters ().
Figure 2 Meta-analysis of the association between APC promoter methylation and the risk of colorectal cancer. The circles and horizontal lines correspond to the study specific OR and 95% CI. The sizes of the data markers indicate the weight of each study in the analysis. The diamond represents the pooled OR and its 95% CI; the result was obtained using a fixed-effect model.
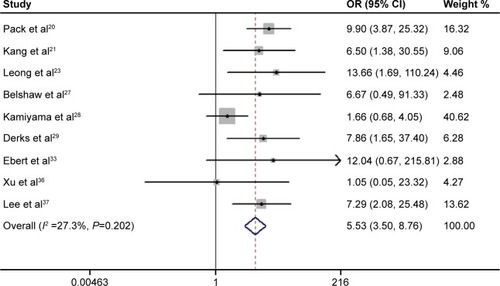
Figure 3 Meta-analysis of the association between APC promoter methylation in samples of colorectal cancer and samples of colorectal cancer metastasis. The circles and horizontal lines correspond to the study specific OR and 95% CI. The sizes of the data markers indicate the weight of each study in the analysis. The diamond represents the pooled OR and its 95% CI; the result was obtained using a random-effect model.
Abbreviations: CI, confidence interval; OR, odds ratio.
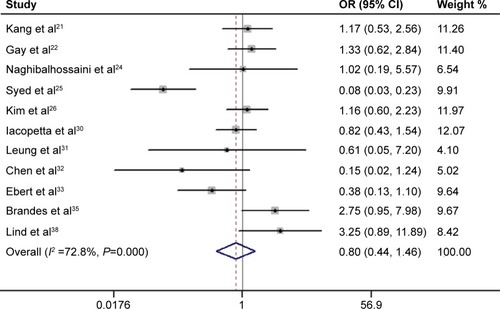
Figure 4 Meta-analysis of the association between APC promoter methylation in samples of colorectal cancer and colorectal adenoma. The circles and horizontal lines correspond to the study specific OR and 95% CI. The sizes of the data markers indicate the weight of each study in the analysis. The diamond represents the pooled OR and its 95% CI; the result was obtained using a random-effect model.
Abbreviations: CI, confidence interval; OR, odds ratio.
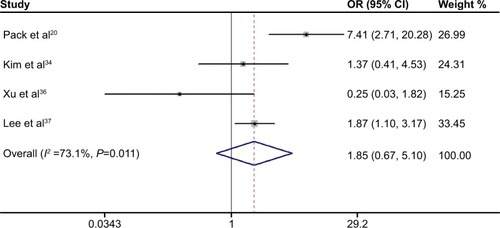
Table 2 Meta-analysis of the association between APC promoter methylation and the clinical characteristics of patients with colorectal cancer
We conducted subgroup analyses to further explore the potential effects of the adopted screening methods, the ethnicities of the study populations, the testing materials used, and the study sample sizes on the association of APC promoter methylation with the risk of CRC (). When the studies were stratified according to ethnicity, the combined OR was 4.54 (95% CI 1.89–10.92; P<0.01) for studies conducted with Asian populations and 10.03 (95% CI 3.44–29.19; P<0.01) for studies conducted with Caucasian populations. Subgroup analyses of the testing materials used suggested that the exposure rate of APC promoter methylation in CRC tissues was higher than that in normal tissues (pooled OR 4.13; 95% CI 2.39–7.15; P<0.01). In addition, a significant correlation between APC promoter methylation and the risk of developing CRC was detected in studies that used both methylation-specific polymerase chain reaction (pooled OR 8.04; 95% CI 4.22–15.32; P<0.01) and Q-MSP (pooled OR 3.07; 95% CI 1.52–6.16; P<0.01). Moreover, the overall outcome was not significantly altered by the sample size in the subgroup analysis.
Table 3 Subgroup analyses of APC promoter methylation and the risk of colorectal cancer stratified according to the previously defined study characteristics
The subgroup analyses, which were stratified according to the methylation screening methods adopted, the ethnicity of the study population, the testing materials used, and the study sample sizes, were conducted to detect potential clinical heterogeneity among the studies that investigated the association between APC promoter methylation and CRC metastasis (). None of these factors significantly affected the overall outcome of the association between APC promoter methylation and CRC metastasis.
Table 4 Subgroup analyses of APC promoter methylation and colorectal metastasis stratified according to previously defined study characteristics
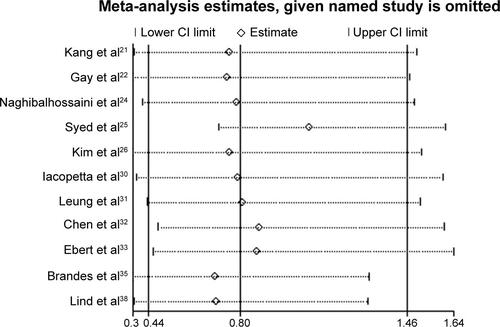
The sensitivity analyses showed that the pooled ORs were not significantly affected following the sequential exclusion of each study (Figures S1–S3). In addition, pooling the data of the high-quality studies during the sensitivity analyses did not significantly change the overall results of the meta-analysis. The funnel plot (Figure S4) and Egger’s test did not reveal significant publication biases in the current meta-analysis (CRC risk, Egger’s test, P=0.48; CRC metastasis status, Egger’s test, P=0.85; colorectal adenoma status, Egger’s test, P=0.85).
Discussion
The main findings of this meta-analysis indicated that APC promoter methylation was significantly associated with a higher risk of developing CRC. Additional subgroup analyses of the adopted methylation screening methods, ethnicities of the study populations, the testing materials used, and the sample sizes did not significantly alter the overall results. Both the overall and subgroup analyses regarding the association between APC promoter methylation and CRC metastasis suggested that APC promoter methylation was not significantly associated with the presence of CRC metastasis. Furthermore, this meta-analysis did not identify a significant association between APC promoter methylation and CRC adenoma, patient age, patient sex, TNM stage, Dukes’ stage, or differentiation grade.
It is well known that CRC has relatively low 5-year survival rate worldwide.Citation1 However, CRC is curable in >90% cases if detected at an early stage.Citation39 Therefore, the development and improvement of early screening methods are essential to increasing the survival rate and clinical outcomes for patients with CRC. In addition, the excision of precancerous polyps under early screening programs is one of the most effective methods used to decrease the incidence and mortality rate of CRC.Citation3,Citation40,Citation41 It has been suggested that the fecal occult blood test, a commonly used screening method for CRC, can decrease CRC-related mortality by 20% when conducted every 2 years;Citation42 however, the fecal occult blood test still has a comparatively low detection rate for early-stage CRC and precancerous lesions.Citation43 Although other screening tests such as colonoscopy, flexible sigmoidoscopy, and virtual colonoscopy are more robust, they are partially limited by their relatively high cost, complex preparation procedures, and low compliance rates in the screening and surveillance of CRC.Citation44 Therefore, the identification of CRC biomarkers has attracted more attention and may hold great potential as a new screening method for the prevention and subsequent treatment of CRC.Citation7
The present meta-analysis indicated that the percentage of APC promoter methylation in specimens of CRC was significantly higher than in normal samples, which is consistent with the result of a previous meta-analysis of APC promoter methylation and prostate cancer.Citation45 These findings suggest that APC promoter methylation may be a valuable biomarker for CRC carcinogenesis. In addition, that methylated DNA can be detected with a high degree of sensitivity and specificity provides evidence that this methodology may be helpful in disease diagnosis and risk stratification.Citation7,Citation46 Thus, testing for APC promoter methylation may be useful as a practical method for CRC screening. However, we did not identify a significant association between APC promoter methylation and CRC metastasis, Dukes’ stage, TNM stage, or differentiation grade, which suggests that this method may be inappropriate for use when predicting the developmental stage of CRC. This needs to be further evaluated by additional high-quality studies.
The evaluation of heterogeneity among studies is an essential requirement when performing a meta-analysis.Citation47 In this meta-analysis, heterogeneity among the studies was detected using the chi-square-based Q-test and the I2 test. Following a systematic assessment, we found no significant heterogeneity between the studies that had investigated the association between APC promoter methylation and the risk of developing CRC. Subgroup analyses were conducted to evaluate the clinical heterogeneity of the included studies that investigated the association between APC promoter methylation and the risk of developing CRC or CRC metastasis, and no significant heterogeneity was found. In addition, sensitivity analyses were performed to explore the effects of individual studies and study quality on each overall outcome, and no single sensitive study was found in this meta-analysis. Publication bias has also been considered a major concern for a robust meta-analysis. Thus, we used the funnel plot and Egger’s test to estimate the influence of publication bias, and no significant publication bias was identified in our study. The aim of a meta-analysis is to aggregate information in order to achieve higher statistical power in the study. However, problems associated with low statistical power may exist even after combining the included studies in the meta-analysis. Therefore, we performed a test to evaluate the statistical power of the pooled results. The power of the positive results of this meta-analysis ranged from 0.96 to 1.0, indicating that these positive results are credible.Citation48,Citation49
The present meta-analysis has several limitations. First, we could not rule out the effects of potential confounders such as xenoestrogens, folate, and diet on the findings of this meta-analysis due to a lack of information presented for both the case and control groups in the studies that were reviewed. Second, the meta-analysis was based on data from observational studies, which may have some potential recall and selection biases that we could not rule out. Third, although no significant publication bias was found in this investigation, null findings may not have been published in the analyzed studies that had considered APC promoter methylation as a secondary outcome. In addition, the power value was lower than 0.8 in part of the overall and subgroup analyses of the meta-analysis with negative results. This indicated that the combined sample size was still too small to yield statistical power, even when we pooled the results of the included studies.Citation48,Citation49 Therefore, these results need to be bolstered by additional high-quality studies with larger sample sizes in the future.
Conclusion
In conclusion, APC promoter methylation was found to be significantly associated with the risk of developing CRC, which suggested that APC promoter methylation may be a promising biomarker for the early screening of CRC. In addition, this meta-analysis did not find a significant association between APC promoter methylation and CRC metastasis, colorectal adenoma status, patient age, patient sex, TNM stage, Dukes’ stage, or differentiation grade. The conclusions of the current study need to be evaluated further through well-designed prospective studies with larger sample sizes. In addition, findings might be more convincing if the studies can effectively rule out the potential effects of various confounding factors.
Author contributions
All authors contributed to the data analysis and the drafting and revising of the paper, and agree to be accountable for all aspects of the work.
Acknowledgments
The study was supported by the subtopics of National Key Drug Discovery Project in the 12th Five-Year Period (grant 2012ZX0903016-002) and by the Liaoning Province Science and Technology Development Funds (grant 2012225019). English language editing of this manuscript was provided by Journal Prep.
Supplementary materials
Figure S1 Sensitivity analyses of the association between APC promoter methylation and the risk of colorectal cancer.
Abbreviation: CI, confidence interval.
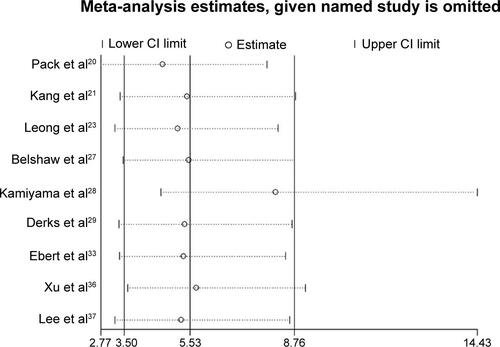
Figure S2 Sensitivity analyses of the association between APC promoter methylation in samples of colorectal cancer and samples of colorectal cancer metastasis.
Abbreviation: CI, confidence interval.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRNaishadhamDJemalACancer statistics, 2013CA Cancer J Clin2013631113023335087
- SilvaTDVidigalVMFelipeAVDNA methylation as an epigenetic biomarker in colorectal cancerOncol Lett2013661687169224260063
- EdwardsBKWardEKohlerBAAnnual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future ratesCancer2010116354457319998273
- DumitrescuRGEpigenetic markers of early tumor developmentMethods Mol Biol201286331422359284
- FearonERMolecular genetics of colorectal cancerAnnu Rev Pathol2011647950721090969
- KojimaMOchiaiAMolecular genetics associated with invasion and metastasis of colorectal cancerNihon Rinsho201169Suppl 3149152 Japanese22213947
- KimMSLeeJSidranskyDDNA methylation markers in colorectal cancerCancer Metastasis Rev201029118120620135198
- EadsCADanenbergKDKawakamiKMethyLight: a high-throughput assay to measure DNA methylationNucleic Acids Res2000288E3210734209
- FoddeRSmitsRCleversHAPC, signal transduction and genetic instability in colorectal cancerNat Rev Cancer200111556711900252
- BehrensJThe role of the Wnt signalling pathway in colorectal tumorigenesisBiochem Soc Trans20053367267516042571
- NarayanSRoyDRole of APC and DNA mismatch repair genes in the development of colorectal cancersMol Cancer200324114672538
- SweeneyCBoucherKMSamowitzWSOncogenetic tree model of somatic mutations and DNA methylation in colon tumorsGenes Chromosomes Cancer2009481918767147
- KumarARajendranVSethumadhavanRPurohitREvidence of colorectal cancer-associated mutation in MCAK: a computational reportCell Biochem Biophys20136783785123564489
- PurohitRSethumadhavanRStructural basis for the resilience of Darunavir (TMC114) resistance major flap mutations of HIV-1 proteaseInterdiscip Sci2009132032820640812
- RajendranVPurohitRSethumadhavanRIn silico investigation of molecular mechanism of laminopathy caused by a point mutation (R482W) in lamin A/C proteinAmino Acids20124360361521989830
- PurohitRRajendranVSethumadhavanRRelationship between mutation of serine residue at 315th position in M. tuberculosis catalase-peroxidase enzyme and Isoniazid susceptibility: an in silico analysisJ Mol Model20111786987720593210
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- StroupDFBerlinJAMortonSCMeta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) groupJAMA2000283152008201210789670
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- PackSCKimHRLimSWUsefulness of plasma epigenetic changes of five major genes involved in the pathogenesis of colorectal cancerInt J Colorectal Dis201328113914722990173
- KangHJKimEJKimBGQuantitative analysis of cancer-associated gene methylation connected to risk factors in Korean colorectal cancer patientsJ Prev Med Public Health201245425125822880157
- GayLJMitrouPNKeenJDietary, lifestyle and clinicopathological factors associated with APC mutations and promoter methylation in colorectal cancers from the EPIC-Norfolk studyJ Pathol2012228340541522864938
- LeongKJWeiWTannahillLAMethylation profiling of rectal cancer identifies novel markers of early-stage diseaseBr J Surg201198572473421360524
- NaghibalhossainiFHosseiniHMMokarramPZamaniMHigh frequency of genes’ promoter methylation, but lack of BRAF V600E mutation among Iranian colorectal cancer patientsPathol Oncol Res201117481982521455633
- SyedSAShahZAAbdullahSChowdriNASiddiqiMAAnalysis of molecular aberrations of Wnt pathway gladiators in colorectal cancer in the Kashmiri populationHum Genomics20115544145221807601
- KimJCChoiJSRohSAChoDHKimTWKimYSPromoter methylation of specific genes is associated with the phenotype and progression of colorectal adenocarcinomasAnn Surg Oncol20101771767177620077021
- BelshawNJPalNTappHSPatterns of DNA methylation in individual colonic crypts reveal aging and cancer-related field defects in the morphologically normal mucosaCarcinogenesis20103161158116320395289
- KamiyamaHNodaHTakataOSuzukiKKawamuraYKonishiFPromoter hypermethylation of tumor-related genes in peritoneal lavage and the prognosis of patients with colorectal cancerJ Surg Oncol20091001697419384904
- DerksSPostmaCMoerkerkPTPromoter methylation precedes chromosomal alterations in colorectal cancer developmentCell Oncol2006285–624725717167178
- IacopettaBGrieuFLiWAPC gene methylation is inversely correlated with features of the CpG island methylator phenotype in colorectal cancerInt J Cancer2006119102272227816981189
- LeungWKToKFManEPQuantitative detection of promoter hypermethylation in multiple genes in the serum of patients with colorectal cancerAm J Gastroenterol2005100102274227916181380
- ChenJRockenCLofton-DayCMolecular analysis of APC promoter methylation and protein expression in colorectal cancer metastasisCarcinogenesis2005261374315375009
- EbertMPMooneySHTonnes-PriddyLHypermethylation of the TPEF/HPP1 gene in primary and metastatic colorectal cancersNeoplasia20057877177816207479
- KimHCRohSAGaIHKimJSYuCSKimJCCpG island methylation as an early event during adenoma progression in carcinogenesis of sporadic colorectal cancerJ Gastroenterol Hepatol200520121920192616336454
- BrandesJCvan EngelandMWoutersKAWeijenbergMPHermanJGCHFR promoter hypermethylation in colon cancer correlates with the microsatellite instability phenotypeCarcinogenesis20052661152115615760919
- XuXLYuJZhangHYMethylation profile of the promoter CpG islands of 31 genes that may contribute to colorectal carcinogenesisWorld J Gastroenterol200410233441345415526363
- LeeSHwangKSLeeHJKimJSKangGHAberrant CpG island hypermethylation of multiple genes in colorectal neoplasiaLab Invest200484788489315122305
- LindGEThorstensenLLovigTA CpG island hypermethylation profile of primary colorectal carcinomas and colon cancer cell linesMol Cancer200432815476557
- ToribaraNWSleisengerMHScreening for colorectal cancerN Engl J Med1995332138618677870142
- CressRDMorrisCEllisonGLGoodmanMTSecular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992–2001Cancer20061075 Suppl1142115216835912
- PhillipsKALiangSYLadabaumUTrends in colonoscopy for colorectal cancer screeningMed Care200745216016717224779
- AtkinWOptions for screening for colorectal cancerScand J Gastroenterol20032371316
- ImperialeTFRansohoffDFItzkowitzSHTurnbullBARossMEFecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk populationN Engl J Med2004351262704271415616205
- GrutzmannRMolnarBPilarskyCSensitive detection of colorectal cancer in peripheral blood by septin 9 DNA methylation assayPLoS One2008311e375919018278
- ChenYLiJYuXAPC gene hypermethylation and prostate cancer: a systematic review and meta-analysisEur J Hum Genet201321992993523299921
- MahoneySEYaoZKeyesCCTapscottSJDiedeSJGenome-wide DNA methylation studies suggest distinct DNA methylation patterns in pediatric embryonal and alveolar rhabdomyosarcomasEpigenetics20127440040822419069
- LauJIoannidisJPSchmidCHSumming up evidence: one answer is not always enoughLancet199835190961231279439507
- CampbellMJJuliousSAAltmanDGEstimating sample sizes for binary, ordered categorical, and continuous outcomes in two group comparisonsBMJ1995311114511487580713
- ChanAWHrobjartssonAJorgensenKJGotzschePCAltmanDGDiscrepancies in sample size calculations and data analyses reported in randomised trials: comparison of publications with protocolsBMJ2008337a229919056791