Abstract
Hypoxia-inducible factor-1 (HIF-1) is a master regulator of the transcriptional response to hypoxia. HIF-1α is one of the most compelling anticancer targets. Andrographolide (Andro) was newly identified to inhibit HIF-1 in T47D cells (a half maximal effective concentration [EC50] of 1.03×10−7 mol/L), by a dual-luciferase reporter assay. It suppressed HIF-1α protein and gene accumulation, which was dependent on the inhibition of upstream phosphatidylinositol 3-kinase (PI3K)/AKT pathway. It also abrogated the expression of HIF-1 target vascular endothelial growth factor (VEGF) gene and protein. Further, Andro inhibited T47D and MDA-MB-231 cell proliferation and colony formation. In addition, it exhibited significant in vivo efficacy and antitumor potential against the MDA-MB-231 xenograft in nude mice. In conclusion, these results highlighted the potential effects of Andro, which inhibits HIF-1, and hence may be developed as an antitumor agent for breast cancer therapy in future.
Introduction
Breast cancer is the most commonly occurring malignancy in women, contributing to approximately 500,000 deaths per year worldwide.Citation1 Hypoxia occurs in breast cancer and in other solid tumors due to the tumor outgrowing the existing vasculature.Citation2 Cancer cells adapt to hypoxic conditions by increasing the levels of hypoxia-inducible factors (HIFs), which induce the expression of multiple genes involved in angiogenesis, cell proliferation, invasion, and metastasis.Citation3–Citation5
HIF-1α expression is important for tumor growth and angiogenesis. One established pathway implicated in HIF protein synthesis is the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) pathway.Citation6 Growth factor ligands (eg, epidermal growth factor, EGF) engage the extracellular domain of their target receptor (eg, epidermal growth factor receptor, EGFR) and initiate a cascade of phosphorylation events via PI3K and AKT. Inhibition of the mTOR pathway suppresses HIF-1α expression and decreases activation of its target genes.Citation7
As a transcription factor, HIF-1 is the master regulator of cellular hypoxic response.Citation8 HIF-1 functions as a heterodimeric protein composed of an O2-regulated HIF-1α subunit and a constitutively expressed HIF-1β subunit.Citation9 Under normal conditions, the HIF-1α subunit is hydroxylated on proline residue, which targets HIF-1α for subsequent proteasomal degradation. Under hypoxia, HIF-1α is quickly stabilized and translocates to the nucleus, where it forms a heterodimer with HIF-1β and subsequently binds to the hypoxia responsive element (HRE), resulting in transactivation of more than 200 genes required for the cell to adapt to hypoxic conditions, including those that code for erythropoietin, vascular endothelial growth factor (VEGF), and various glycolytic enzymes.Citation10 Importantly, the vast majority of these gene products are overexpressed in human tumor cells, suggesting that the HIF-dependent transcription changes are important in tumor pathophysiology. Thus, HIF-1α is viewed as an excellent target for the development of novel cancer therapeutics. Compounds that regulate hypoxic events, especially HIFs, are therefore proposed to represent useful targets for anticancer therapy.Citation11
Andrographis paniculata is a traditional herbal medicine used in Southeast Asian countries. Diterpene lactone is the major component of Andrographolide (Andro) and constitutes 70% of the plant extract.Citation12 Andro has been reported to have multiple pharmacological properties and has been widely used in clinic for the treatment of fever, cold, inflammation, diarrhea, and other infectious diseases.Citation13,Citation14 Recent studies suggest that Andro possesses antitumor activity, and various mechanisms are involved. Andro can inhibit cell cycle progression, reduce cell invasion, or induce cell apoptosis by targeting different target genes in different cancer cells.Citation15,Citation16 This study aims to unravel the involvement of HIF-1 and its upstream PI3K/AKT signaling in Andro’s antitumor effect in human breast cancer.
Materials and methods
Cell culture
Human breast cancer cell lines T47D (cultured in RPMI-1640) and MDA-MB-231 (cultured in Leibovitz’s L-15 medium) were obtained from ATCC. The medium contains 10% fetal bovine serum (Hyclone Laboratories Inc, UT, USA), 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, Thermo Fisher Scientific, Waltham, MA, USA). The cell lines were maintained at 37°C in a humidified incubator containing 21% O2 and 5% CO2. For the hypoxia experiment, cells were treated with Andro first and then incubated under 1% O2, 94% N2, and 5% CO2. Andro (purity 98%) was purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA).
Dual-luciferase reporter assay
T47D or MDA-MB-231 cells seeded in 96-well plates were cotransfected with 0.01 mg pRL-CMV (Promega Corporation, Fitchburg, WI, USA) and 0.2 mg pGL2-TK-HRE by lipofectamine 2000 (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) as described elsewhere.Citation17 pGL2-TK-HRE plasmid carried a firefly luciferase gene driven by three tandem repeats of HRE sequences. About 24 hours later, the cells were treated with compounds for 0.5 hours, then moved to a hypoxia chamber and incubated for another 16 hours for dual luciferase activity assays (Promega Corporation).
Western blotting assay
Cells were lysed by RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, 0.1% SDS (sodium dodecyl sulfate), and protease inhibitor cocktails (Amresco, Solon, OH, USA). Equal amounts of proteins were separated by SDS-PAGE (poly-acrylamide gel electrophoresis) gels and transferred to PVDF (polyvinylidene difluoride) membranes (Millipore, Billerica, MA, USA). Membranes were blocked with 5% milk, followed by incubation with primary antibodies and then horseradish peroxidase (HRP)-conjugated secondary antibodies for protein visualization. All the primary antibodies were purchased from CST (Beverly, MA, USA).
Real-time reverse transcription–PCR
Total RNAs were isolated by the Trizol (Invitrogen) method, and then the mRNAs were reverse-transcribed into cDNAs by an iScript cDNA synthesis kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). Real-time reverse transcription–PCR (RT-qPCR) was performed using a SYBR supermix kit (Bio-Rad Laboratories Inc.) following the manufacturer’s protocol with ABI PRISM 7900 HT sequence detector. The PCR efficiency was examined by a serial dilution of the cDNA, and PCR specificity was checked by melting curve data. Each cDNA sample was triplicated, and the corresponding no-RT mRNA sample was included as a negative control. The primers of GAPDH were included in every plate to avoid sample variations. The mRNA level of each sample for each gene was normalized to that of GAPDH mRNA. Primers were as follows:
5′-CACCCTCTTCGTCGCTTCGG-3′ (HIF-1α forward) and 5′-GCGGGAAACCCCTCGTGAGA-3′ (HIF-1α reverse), 5′-TACCTCCACCATGCCAAGTG-3′ (VEGF forward) and 5′-ATGATTCTGCCCTCCTCCTTC-3′ (VEGF reverse).
ELISA and MTT assay
T47D cells seeded in 96-well plates were treated with Andro, and 16 hours later, the medium was collected for testing. VEGF amounts were determined following the manufacturer’s instructions (R&D Systems, Inc., Minneapolis, MN, USA) by enzyme-linked immunosorbent assay (ELISA). For MTT cell viability assay, the cells were seeded at a density of 1,500 cells per well. After treatment with the compound for 5 days, the cell viability was tested following the manufacturer’s protocol (Biotium, Hayward, CA, USA). IC50 values (50% inhibitory concentration) were calculated using the software GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA).
In vivo study
The human breast cancer cells (MDA-MB-231) were grown and harvested. Cells were resuspended in saline at 10 million cells/0.2 mL and placed on ice. Nude (BALB/c females, 6–8 weeks old) mice were injected (SC) with 0.2 mL of this suspension on the right flank and observed daily for tumor appearance. When the tumors attained a volume of 100–200 mm3, they were randomized into five groups. For control, 0.5% CMC-Na was administered every day PO for 24 days. Andro (25, 50, and 100 mg/kg), which was dissolved in 0.5% CMC-Na, was administered every day PO also for 24 days. As a positive control, Taxol (30 mg/kg) was injected intraperitoneal (IP) twice weekly. The nude mice were monitored every day, and the tumor volume was estimated by measuring the tumor size twice a week using the following formula: tumor volume =0.5×L×W2, where L and W represent the largest diameter and the smallest diameter, respectively. After the final treatment, the animals were sacrificed and the tumors were removed and weighted.
Statistical analysis
Statistical analysis was made using the GraphPad PRISM® (version 5.0, GraphPad Software, Inc.) software. Student’s unpaired t-test was employed for the comparison. Statistical significance was evaluated by calculating P-values. Differences where P<0.05 were considered statistically significant. (*P<0.05, **P<0.01, ***P<0.001). Densitometric analysis was carried out using the ImageJ software.
Results
Andro inhibited HIF-1
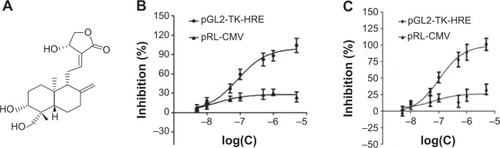
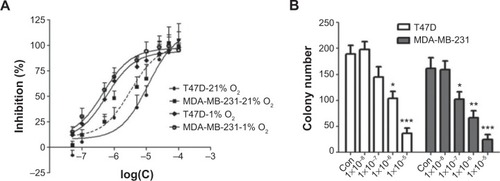
A cell-based reporter assay was used to monitor HIF-1 activity or protein expression level. Specificity index was calculated, obtained by dividing the half maximal effective concentration (EC50) of pRL-CMV by the EC50 of pGL2-TK-HRE, which provides an indication of relative specificity toward inhibition of HIF-1. Among more than 100 natural products, Andro () was screened out to specifically inhibit HIF-1. Andro had a specificity index >100 in both cell lines, indicating that it had a strong effect on firefly luciferase (EC50 of pGL2-TK-HRE in T47D cells =1.03×10−7 mol/L, in MDA-MB-231 cells =7.74×10−8 mol/L), but had minimal activity on the constitutive expression of ranilla luciferase (EC50 of pRL-CM in both cell lines >10−5 mol/L) (). As HIF-1α protein is overexpressed in most breast cancer cell lines, it was mainly used for further studies.
Figure 1 Andro specifically inhibited HIF-1 in T47D and MDA-MB-231 cells.
Abbreviations: Andro, Andrographolide; HIF-1, hypoxia-inducible factor-1.
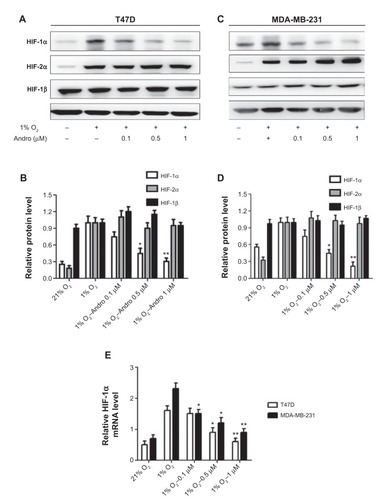
Andro blocked hypoxia-induced HIF-1α accumulation
The ability of Andro to inhibit HIF-1 expression was tested by evaluating the HIF-1 expression levels in the breast cancer cells T47D and MDA-MB-231 under hypoxia. As shown in , hypoxia induced HIF-1α expression in both T47D and MDA-MB-231 cells, and incubation with Andro (8 hours) led to a dose-dependent decrease of HIF-1α, but not HIF-2α or HIF-1β. The HIF-1α protein level was reduced to 0.3-fold (T47D) and 0.2-fold (MDA-MB-231) in the 1% O2 group (P<0.01) after 1 μM Andro treatment. To elucidate whether this decrease in HIF-1α protein level was caused by the effect of Andro on mRNA expression under our experimental conditions, qPCR assay was employed. Indeed, the addition of Andro significantly reduced HIF-1α mRNA level in a dose-dependent manner under hypoxia (). Thus, Andro blocks the hypoxia-induced accumulation of HIF-1α at the transcriptional stage by reducing HIF-1α mRNA level.
Figure 2 Andro specifically inhibited HIF-1α protein and gene accumulation under hypoxia.
Abbreviations: Andro, Andrographolide; HIF-1, hypoxia-inducible factor-1; SD, standard deviation; mRNA, messenger RNA; qPCR, real-time polymerase chain reaction.
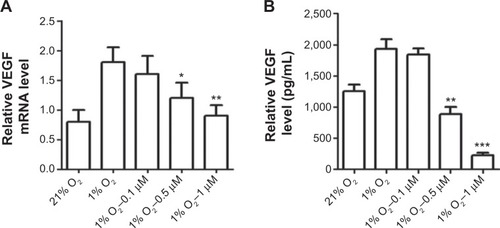
Andro downregulated HIF-1α-mediated VEGF expression
Increased HIF-1a expression promotes transcriptional activation of many genes. VEGF is a prime candidate.Citation18 Thus, we determined the levels of VEGF expression, which was upregulated by hypoxia-induced HIF-1 transcriptional activation. Andro treatment significantly inhibited hypoxia-induced VEGF mRNA level (). Furthermore, the VEGF protein level was also reduced by Andro in a dose-dependent manner ().
Figure 3 Andro decreased HIF-1 target protein VEGF and its mRNA expression.
Abbreviations: Andro, Andrographolide; HIF-1, hypoxia-inducible factor-1; SD, standard deviation; VEGF, vascular endothelial growth factor; ELISA, enzyme-linked immunosorbent assay; mRNA, messenger RNA; qPCR, real-time polymerase chain reaction.
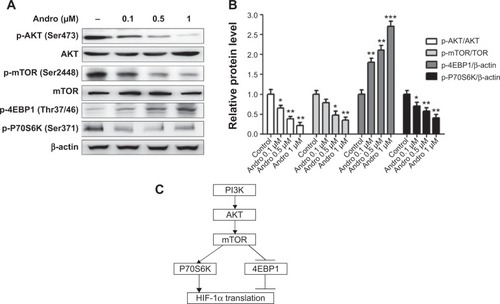
Andro inhibited PI3K signaling pathway under hypoxia
Translation of HIF-1α mRNA has been previously suggested to be under the control of the PI3K signaling pathway in various cell types ().Citation19,Citation20 To address whether Andro affected HIF-1α upstream PI3k/AKT/mTOR pathway, we performed experiments in MDA-MB-231 cells, which constitutively expressed phosphorylation of mTOR, 4EBP1, and P70S6K under hypoxia. As shown in , Andro decreased the phosphorylation level of AKT, mTOR and p70s6k. Meanwhile, 4EBP1 phosphorylation was induced by Andro in a dose-dependent manner. These results indicated that Andro suppresses HIF-1α activity probably via the inhibition of the upstream PI3K/AKT/mTOR pathway.
Figure 4 Inhibition of HIF-1α accumulation by Andro is dependent on the PI3K/AKT/mTOR pathway.
Abbreviations: Andro, Andrographolide; HIF-1, hypoxia-inducible factor-1; SD, standard deviation.
Andro decreased breast cancer cell growth in vitro
HIF-1 is an antitumor target.Citation21 To test whether Andro’s inhibitory effect on HIF-1 would affect cancer cell viability, MTT and colony formation assays were performed in breast cancer cell lines. As shown in , Andro suppressed cell proliferation in a dose-dependent manner, with an IC50 of 11.3 μM in T47D and 3.2 μM in MDA-MB-231 under 21% O2, whereas under 1% O2, Andro had a more potent inhibitory effect on cancer cell growth (an IC50 of 1.2 μM in T47D and 0.86 μM in MDA-MB-231). The results also demonstrated that Andro significantly inhibited the cell colony formation of T47D and MDA-MB-231cells at a very low concentration ().
Figure 5 In vitro antitumor effect of Andro on breast cancer cells.
Abbreviation: Andro, Andrographolide.
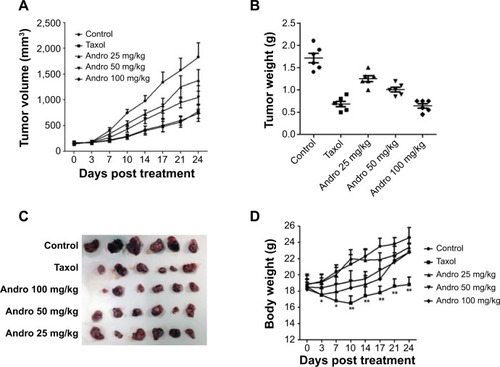
Andro inhibited tumor xenograft growth in vivo
Following the in vitro antitumor effects of Andro, to evaluate the antitumor activity of Andro in vivo, we implanted human breast MDA-MB-231 tumors in nude mice and then treated the nude mice with 25, 50, and 100 mg/kg of Andro (every day) via oral administration. As a positive control, Taxol was given to mice twice a week. Andro significantly inhibited the growth of these tumor xenografts in a dose-dependent manner (). Not only tumor volumes () but also tumor weights () were significantly decreased by Andro. The average tumor weight on day 24 in the control group was 1.72±0.26 g, and the tumor weights in the experimental groups (25, 50, and 100 mg/kg of Andro) were 1.25±0.16, 1.01±0.12, and 0.68±0.15 g, respectively. The calculated final percentage of growth inhibition was observed to be 27.2%, 41.3%, and 60.2% (). Mice treated with Andro had no significant weight loss (). These results indicate that Andro effectively inhibits the tumor growth in vivo with very low toxicity.
Figure 6 Andro suppressed the MDA-MB-231 xenograft tumor growth.
Abbreviations: Andro, Andrographolide; IP, intraperitoneal.
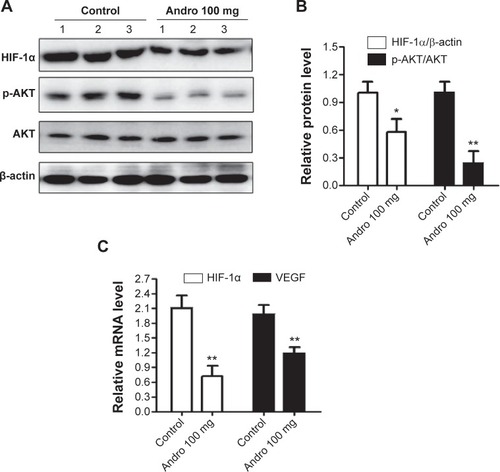
Andro reduced HIF-1α expression, which is dependent on PI3K/AKT/mTOR pathway in vivo
To further confirm Andro’s effect on HIF-1 in vivo, we evaluated HIF-1α levels in MDA-MB-231 tumors using Western blotting and qPCR assays. In accordance with in vitro results, HIF-1α accumulation was significantly reduced in the Andro treatment group (). We also observed that the high expression of HIF-1α and VEGF mRNA in MDA-MB-231 tumors were blocked in the Andro treatment group (). The phosphorylation of AKT protein was also inhibited by Andro (). These in vivo results confirmed that Andro’s antitumor effect is probably via the inhibition of HIF-1α activity and its upstream PI3k/AKT/mTOR pathway.
Figure 7 Andro inhibits HIF-1α accumulation and AKT activity in vivo.
Abbreviations: Andro, Andrographolide; HIF-1, hypoxia-inducible factor-1; SD, standard deviation; VEGF, vascular endothelial growth factor.
Discussion
Andro, whose major component is diterpene lactone, has been shown to possess antitumor properties, and various mechanisms have been reported. For example, Andro suppresses cancer cell proliferation,Citation22 induces cell-cycle arrest,Citation23 and promotes apoptosis.Citation15 The proapoptotic effect of Andro was shown to increase the expression level of Bax in human leukemia HL-60 cells.Citation24 In addition, Andro inhibits the migration and invasion of human non–small cell lung cancer A549 cells via downregulation of PI3K/AKT signaling.Citation25 And it has been newly reported that Andro inhibits these cancer cells by downregulation of HIF-1α expression.Citation26 However, the detailed mechanism is not yet illustrated. On the basis of our luciferase reporter assay, Andro specifically inhibited HIF-1 in breast cancer cells. Since the HIF-1 pathway can be inhibited at several different stages, including posttranslational modification, gene transcription, or protein translation,Citation27 we investigated whether Andro had any effect on the synthesis of HIF-1α protein or mRNA. First, Andro’s effect on HIF-1α protein level in breast cancer cells was checked. Andro specifically suppressed HIF-1α protein under hypoxia in a dose-dependent manner but not HIF-2α and HIF-1β. Next, our results indicated that the inhibition of HIF-1α expression by Andro was due to its inhibitory effect on HIF-1α mRNA levels. As a HIF-1 target gene, inhibition of VEGF mRNA and protein was observed in T47D and MDA-MB-231 breast cancer cells after HIF-1α inhibition.
The PI3K/AKT pathway plays a key role in the control of HIF-1α translation and synthesis in certain types of cancer cells.Citation7 The possibility of inhibition of HIF-1α synthesis by Andro from its upstream PI3K/AKT pathway was therefore considered. Treatment of hypoxic MDA-MB-231 cells with Andro resulted in reduced HIF-1α expression as well as decreased phosphorylation of AKT, mTOR, and its downstream effector P70S6K, but increased phosphorylation of 4EBP1, which controls the initiation of protein translation under hypoxiaCitation28 in a dose-dependent manner. These observations support the hypothesis that Andro-mediated HIF-1α inhibition was dependent on PI3K/AKT pathway by inhibition of HIF-1α translation.
As a HIF-1 inhibitor, Andro not only suppressed the upstream of PI3K pathway but also decreased the downstream VEGF protein level. Since HIF-1, PI3K, and VEGF are all well-known antitumor targets, next we tested Andro’s antitumor effect in vitro and in vivo. Andro suppressed the breast cancer cells’ proliferation. A number of antitumor compounds have been reported to decrease HIF-1α activity; however, only a few of the reported inhibitors demonstrated antitumor activity in vivo.Citation29 Because of the promising in vitro results from MTT and colony formation assays, we examined the tumor growth inhibition effect by Andro. The human MDA-MB-231 xenograft model showed antitumor efficacy of Andro in breast cancers. Notably, the antitumor activity of Andro is associated with a decrease in HIF-1α mRNA and protein levels, a decreased expression of the downstream targets of VEGF and the upstream AKT from the in vivo studies.
In summary, all the results suggest that Andro is a novel inhibitor of HIF-1 that halts tumor growth by blocking tumor adaptation to hypoxia and thus can be used as a therapeutic agent for breast cancer.
Disclosure
The authors report no conflicts of interest in this work.
References
- MilaniMHarrisALTargeting tumour hypoxia in breast cancerEur J Cancer2008442766277318990559
- GilkesDMSemenzaGLRole of hypoxia-inducible factors in breast cancer metastasisFuture Oncol201391623163624156323
- SemenzaGLHIF-1 and human disease: one highly involved factorGenes Dev2000141983199110950862
- Brahimi-HornMCPouyssegurJThe hypoxia-inducible factor and tumor progression along the angiogenic pathwayInt Rev Cytol200524215721315598469
- ShannonAMBouchier-HayesDJCondronCMTumour hypoxia, chemotherapeutic resistance and hypoxia-related therapiesCancer Treat Rev20032929730712927570
- SemenzaGLHIF-1 mediates metabolic responses to intratumoral hypoxia and oncogenic mutationsJ Clin Invest20131233664367123999440
- JiangBHLiuLZAKT signaling in regulating angiogenesisCurr Cancer Drug Targets20088192618288940
- HarrisALHypoxia–a key regulatory factor in tumour growthNat Rev Cancer20022384711902584
- WangGLJiangBHRueEAHypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tensionProc Natl Acad Sci U S A199592551055147539918
- SemenzaGLHypoxia, clonal selection, and the role of HIF-1 in tumor progressionCrit Rev Biochem Mol Biol2000357110310821478
- MelilloGInhibiting hypoxia-inducible factor 1 for cancer therapyMol Cancer Res2006460160516940159
- ZhaoJYangGLiuHDetermination of andrographolide, deoxyandrographolide and neoandrographolide in the Chinese herb Andrographis paniculata by micellar electrokinetic capillary chromatographyPhytochem Anal20021322222712184176
- ReddyVLReddySMRavikanthVA new bis-andrographolide ether from Andrographis paniculata nees and evaluation of anti-HIV activityNat Prod Res20051922323015702635
- ShenYCChenCFChiouWFAndrographolide prevents oxygen radical production by human neutrophils: possible mechanism(s) involved in its anti-inflammatory effectBr J Pharmacol200213539940611815375
- ZhouJZhangSOngCNCritical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cellsBiochem Pharmacol20067213214416740251
- RajagopalSKumarRADeeviDSAndrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculataJ Exp Ther Oncol2003314715814641821
- LangLLiuXLiYA synthetic manassantin a derivative inhibits hypoxia-inducible factor 1 and tumor growthPLoS One20149e9958424925080
- ForsytheJAJiangBHIyerNVActivation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1Mol Cell Biol199616460446138756616
- TreinsCGiorgetti-PeraldiSMurdacaJInsulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathwayJ Biol Chem2002277279752798112032158
- LaughnerETaghaviPChilesKHER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expressionMol Cell Biol2001213995400411359907
- OnnisBRapisardaAMelilloGDevelopment of HIF-1 inhibitors for cancer therapyJ Cell Mol Med2009132780278619674190
- SatyanarayanaCDeeviDSRajagopalanRDRF 3188 a novel semi-synthetic analog of andrographolide: cellular response to MCF 7 breast cancer cellsBMC Cancer200442615207007
- ShiMDLinHHLeeYCInhibition of cell-cycle progression in human colorectal carcinoma Lovo cells by andrographolideChem Biol Interact200817420121018619950
- CheungHYCheungSHLiJAndrographolide isolated from Andrographis paniculata induces cell cycle arrest and mitochondrial-mediated apoptosis in human leukemic HL-60 cellsPlanta Med2005711106111116395645
- LeeYCLinHHHsuCHInhibitory effects of andrographolide on migration and invasion in human non-small cell lung cancer A549 cells via down-regulation of PI3K/Akt signaling pathwayEur J Pharmacol2010632233220097193
- LinHHTsaiCWChouFPAndrographolide down-regulates hypoxia-inducible factor-1alpha in human non-small cell lung cancer A549 cellsToxicol Appl Pharmacol201125033634521134392
- AprelikovaOChandramouliGVWoodMRegulation of HIF prolyl hydroxylases by hypoxia-inducible factorsJ Cell Biochem20049249150115156561
- FukudaRHirotaKFanFInsulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cellsJ Biol Chem2002277382053821112149254
- PatiarSHarrisALRole of hypoxia-inducible factor-1alpha as a cancer therapy targetEndocr Relat Cancer200613Suppl 1S61S7517259560
