Abstract
Epoetin beta belongs to the class of erythropoiesis-stimulating agents (ESAs) that are currently available to treat anemic patients receiving chemotherapy. Chemotherapy-induced anemia affects a high percentage of cancer patients and, due to its negative effects on disease outcome and the patient’s quality of life, should be treated when first diagnosed. Initial trials with ESAs have shown efficacy in improving quality of life and reducing the need for blood transfusions in patients with chemotherapy-induced anemia. However, recent meta-analyses have provided conflicting data on the impact of ESAs on survival and tumor progression. Here we provide an overview of these recent data and review the role of epoetin beta in the treatment of chemotherapy-induced anemia over the past 20 years.
Introduction
Anemia, defined as a reduction in hemoglobin concentration to non-physiological levels, frequently occurs in cancer patients, in particular as a consequence of chemotherapy (chemotherapy-induced anemia, CIA), and may negatively impact their disease outcome and quality of life.Citation1 In 2004, a large European survey found that, at enrollment, 39.3% of all patients and 50.5% of those undergoing chemotherapy had hemoglobin <12 g/dL.Citation2 Frequency, defined as the number of individuals who were anemic at least once during the 6-month study, was shown to be 67% for all patients (39.3% of whom with hemoglobin <10 g/dL) and 75% for those treated with a chemotherapy regimen, being higher in lung and gynecological cancers (77% and 81.4%, respectively). In particular, it ranged from <1% in patients with metastatic breast cancer treated with the combination of 5-fluorouracil-doxorubicin-cyclophosphamide-methotrexate, to 80% in patients on a high-dose cyclophosphamide-mitoxantrone-etoposide regimen.Citation1 Several studies also described severe/life-threatening CIA in 3%–6% of patients with colorectal cancer following different chemotherapy regimens () and in 20%–42% of patients with small cell lung cancer (). With regard to the prevalence of anemia, the highest was observed among patients with leukemia, lymphoma/myeloma, and gynecological cancers ().Citation1
Figure 1 Hemoglobin level of patients at enrollment according to tumor type.
Abbreviation: GI, gastrointestinal.
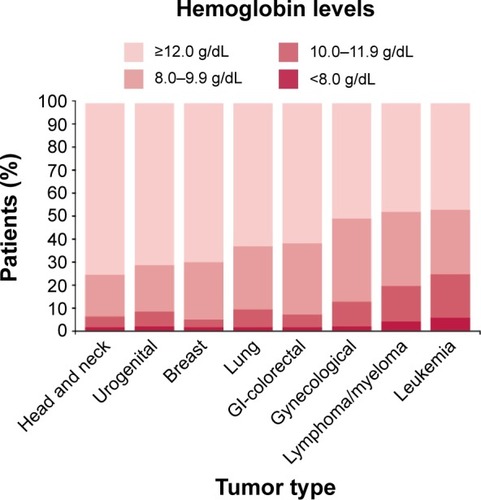
Table 1 Colorectal carcinoma: chemotherapy-induced anemia
Table 2 Small cell lung cancer: chemotherapy-induced anemia
As a consequence of chemotherapy, anemia may develop either by direct impairment of red blood cell (RBC) production or because of renal tubular damage following treatment with cytotoxic drugs.Citation3,Citation4 Other factors causing anemia in cancer patients are linked to tumor growth and development, eg, bleeding, hemolysis, bone marrow infiltration, and disruption of erythropoietin synthesis in the kidney, as a result of increased production of cytokines (eg, interferon-γ, interleukin-1, and tumor necrosis factor-α).Citation5 A reduction in erythropoietin levels negatively affects RBC production in the bone marrow, inducing anemia;Citation6 accordingly, cancer patients with anemic have been shown to present abnormal erythropoietin levels.Citation7
Different strategies were adopted in the past to treat CIA, including allogeneic RBC transfusion, intravenous iron supplementation, and administration of erythropoiesis-stimulating agents (ESAs), the last two often used in combination. Here, we focus on the use of ESAs, particularly epoetin beta, in the management of CIA, and review data on its efficacy and safety. In addition, contrasting findings concerning the impact of epoetin beta on survival and progression are summarized.
Impact of anemia on quality of life and survival in cancer patients
The severity of anemia is variable and can be classified according to toxicity criteria. Two of the most common grading systems are those published by the National Cancer Institute and the World Health Organization ().Citation1 Both agree on classifying severe and life-threatening anemia as hemoglobin levels in the range of 6.5–7.9 g/dL and <6.5 g/dL, respectively; yet, for mild and moderate levels, differences can be observed. The National Cancer Institute classifies moderate anemia as hemoglobin levels of 8–10 g/dL and mild anemia as values between 10 g/dL and normal limits, while the World Health Organization indicates 8–9.4 g/dL and 9.5–10.9 g/dL as moderate and mild anemia, respectively ().
Table 3 Toxicity grading systems for anemia (hemoglobin levels in g/dL)
Anemia affects nearly all major physiological systems, and symptoms may range from cold skin, dizziness, and palpitations to pulmonary edema, heart failure, depression, severe impairment of cognitive function, and fatigue, which can directly impact upon quality of life for patients.Citation1,Citation8 Fatigue is one of the most debilitating symptoms of anemia and an important issue in cancer management, as it is the symptom most commonly reported by cancer patients,Citation9 who feel that fatigue has a worse impact than pain on their quality of life.Citation9,Citation10 A high percentage of oncologists overlook this symptom even if current guidelines recommend routine screening and management of fatigue.Citation9,Citation11 A study conducted in anemic patients, non-anemic cancer patients, and the general population based on a questionnaire from the Functional Assessment of Chronic Illness Therapy Measurement System showed that the worst fatigue scores corresponded to patients in the anemic cancer group.Citation12 The authors further observed that the degree of anemia predicts the degree of fatigue in cancer patients, thus establishing a connection between low hemoglobin levels and increased fatigue.Citation12
In addition to a negative effect on quality of life for cancer patients, anemia has been shown to affect survival and to be an independent prognostic factor for both treatment response and survival in subjects treated with chemotherapy or radiotherapy.Citation13,Citation14 Indeed, from a comprehensive literature review, it emerged that anemia increases the mortality risk in cancer patients by 65%.Citation15 Anemic patients also showed reduced overall survival, disease-free survival, and relapse-free survival, compared with patients who had normal hemoglobin levels.Citation15 Subsequent studies have also demonstrated that anemia is one of the prognostic factors associated with short survival.Citation16,Citation17 Patients developing anemia (hemoglobin <12 g/dL) during chemotherapy had significantly lower overall survival compared with non-anemic patients,Citation18 while the prechemotherapy hemoglobin level is an independent predictor of progression-free survival and overall survival in ovarian cancer.Citation19 A hemoglobin level <10.5 g/dL is also an adverse prognostic factor for disease progression in Hodgkin’s disease.Citation20
Management of CIA
The negative impact of cancer-related anemia, and CIA in particular, on well-being and survival of cancer patients argues for the treatment of this comorbidity with the best therapies available. Until the late 1980s, the only therapy for anemia was RBC transfusion, which although being efficient in rapidly increasing hemoglobin levels in patients, implies high risks of clinical complications.Citation21 Currently, RBC production in the bone marrow can be enhanced using ESAs, erythropoietin-mimicking molecules that activate the erythropoietin receptor (EpoR) on erythrocytic progenitor cells.Citation22,Citation23 In clinical trials, ESAs have been shown to increase hemoglobin levels and improve anemia and quality of life in cancer patients.Citation24–Citation28 Of note, treatment with ESAs was able to reduce the number of RBC transfusions and decrease fatigue in CIA patients.Citation29 However, recent meta-analyses have highlighted possible safety issues regarding ESA exposure. Indeed, results from three large systematic reviews have shown increased mortality in patients receiving these agents.Citation30–Citation32 Subsequent meta-analyses by Ludwig et alCitation33 and Glaspy et alCitation34 have not confirmed these data, as both studies failed to demonstrate a significant effect of ESAs on survival or disease progression. In addition, a few randomized clinical trials have analyzed the effect of ESA treatment on CIA patients and found no decrease of survival.Citation35–Citation37 However, collectively, these studies agree on the possibility of an increased risk of thromboembolic events upon ESAs. According to Bennett et al the risk of thromboembolic events in patients treated with erythropoietin and darbepoetin was 1.57 times higher than in the control group.Citation30 Such risk, however, seemed to depend on hemoglobin levels and not on the disease itself. It is therefore necessary not to exceed the hemoglobin levels recommended by guidelines, since the incidence of adverse events associated with ESAs (especially thromboembolic and cardiovascular events) is higher in patients with hemoglobin ≥13 g/dL.Citation30 To clarify the role of ESAs on disease progression, Aapro recently reviewed published preclinical and clinical evidence and concluded that the available data do not argue for a role of ESAs in tumor progression, even if further studies are needed to clarify whether these effects can occur in certain subgroups of cancer patients.Citation38 However, uncertainties regarding the safety profile of ESAs have prompted international clinical oncology groups to update current guidelines for their use in the treatment of anemia.Citation3,Citation39
Guidelines for use of ESAs
While the efficacy of ESAs in enhancing hemoglobin levels in CIA patients is unquestionable, results for their safety are contradictory and point to the need for additional trials. The current National Comprehensive Cancer Network guidelines recommend to investigate the causes of anemia in cancer patients with hemoglobin levels ≤11 g/dL or ≥2 g/dL below the baseline.Citation3 Following evaluation of risks/benefits and patient agreement, ESAs may be used in CIA patients undergoing palliative treatment, with the exception of patients with small cell lung cancer, given that the clinical data do not show an impact of these agents on disease progression.Citation3 The guidelines from the American Society of Clinical Oncology/American Society of Hematology state that until further safety data are collected, ESAs should be avoided in patients being treated with curative intent.Citation39 For treatment of CIA (hemoglobin <10 g/dL), ESAs may be used but potential harm and benefit should be carefully evaluated.Citation39 For asymptomatic patients with risk factors for development of anemia and requiring transfusions, the clinical options are observation or treatment with ESAs if hemoglobin levels are ≤10 g/dL. Recommended doses are 10,000 IU three times weekly for epoetin alfa and beta, 30,000 IU once weekly for epoetin beta or 40,000 IU once weekly for epoetin alfa, or 150 μg per week or 500 μg every 3 weeks for darbepoetin.Citation40,Citation41 Administration of ESAs involves a high demand for iron as a consequence of the considerably increased erythropoiesis, which may result in RBC production exceeding iron mobilization from stores.Citation42,Citation43 Therefore, assessment of iron status is crucial for a proper therapeutic strategy.
According to the European Medicines Agency’s Committee for Medicinal Products for Human Use, the benefits of using ESAs in approved indications (hemoglobin target range of 10–12 g/dL in CIA) continue to outweigh the associated risks, including increased risk of tumor progression and venous thromboembolism and reduced survival.Citation44 The guidelines do not provide indications on which ESA to use, since the three molecules on the market (epoetin alfa, epoetin beta, and darbepoetin) are considered equivalent in terms of activity, efficacy, and side effects.Citation45
Epoetin beta
Epoetin beta is a human recombinant erythropoietin synthesized in Chinese Hamster Ovary cells, with identical amino acid structure, similar molecular weight, and equivalent characteristics to the human endogenous hormone.Citation46–Citation48 This glycoprotein is composed of an amino acid chain of 156 residues identical to that of the endogenous erythropoietin, and of four carbohydrate side chains that represent approximately 40% of the molecular weight.Citation49 Since 1990, epoetin beta has been licensed in Europe for the treatment of symptomatic anemia associated with chronic kidney disease and to treat CIA in cancer patients.Citation44
Mode of action
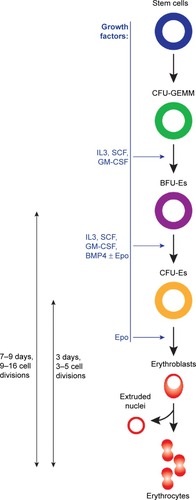
Epoetin beta mimics the mechanism of action of endogenous erythropoietin and has the same biological activity.Citation50 By binding to the EpoR on erythroid progenitors in the bone marrow, epoetin beta stimulates erythropoiesis, the process by which hematopoietic stem cells differentiate into erythrocytes ().Citation6 However, EpoR may be expressed also on the surface of non-hematopoietic cells, of normal and malignant origin.Citation51,Citation52 This implies that, for example, the erythropoietin secreted by cancer cells binds to the EpoR expressed on endothelial cells, causing their proliferation and consequently increased angiogenesis which, in turn, promotes proliferation of tumor cells.Citation53–Citation55 Indeed, preclinical studies have pointed towards the role of erythropoietin in augmenting tumorigenesis, metastatization, and drug resistance at least in certain tumor types (eg, breast cancer), as it can activate important pathways (eg, AKT, ERK, JAK/STAT, antiapoptotic) targeted by current antineoplastic therapies, thus counteracting their effects.Citation56–Citation60
Figure 2 Schematic representation of the erythropoiesis process.
Abbreviations: CFU-GEMM, colony forming unit-granulocyte, erythrocyte, monocyte, megakaryocyte; BFU-E, burst-forming unit-erythroid; CFU-E, colony forming unit-erythroid; IL, interleukin; GM-CSF, granulocyte macrophage-colony stimulating factor; SCF, stem cell factor; BMP, bone morphogenetic protein; Epo, erythropoietin.
A pharmacokinetic and pharmacodynamic evaluation of epoetin beta in healthy volunteers and uremic patients revealed a plasma elimination half-life of 12–28 hours after subcutaneous administration, compared with 4–12 hours after intravenous administration.Citation61 The maximum serum concentration of subcutaneous epoetin beta was reached by 12–18 hours in patients with uremia, with a range of bioavailability between 23% and 52%. Although reduced bioavailability and clearance were observed in patients compared with healthy volunteers, the half-life after subcutaneous administration was similar.Citation61
The exact elimination route is still unknown (<5% is excreted by renal clearance after intravenous administration [10–1,000 IU/kg] in healthy volunteers).Citation62 However, endogenous erythropoietin as well as recombinant products are supposed to be degraded after EpoR-mediated cellular uptake (mainly in the bone marrow), and this could be the main route of elimination from the circulation.Citation63
Clinical efficacy
In cancer patients with CIA, the efficacy of epoetin beta in increasing hemoglobin levels has been demonstrated in numerous trials. In a multicenter, randomized, double-blind, controlled trial, 349 anemic patients (hemoglobin <10 g/dL) with low-grade non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, or multiple myeloma received either epoetin beta 150 IU/kg or placebo subcutaneously three times weekly for 16 weeks.Citation26 The majority of treated patients (67%) showed an increase in hemoglobin levels by 2 g/dL, while only 27% of patients in the placebo group had a similar outcome (P<0.0001); notably, quality of life was significantly better in the epoetin beta group (P<0.05).Citation26
The randomized NOW trial compared the effect of a new dose regimen of epoetin beta (30,000 IU once a week) with that of the established dose of 10,000 IU three times a week, for 16 weeks in anemic patients (n=241, hemoglobin 9–11 g/dL) with lymphoid malignancies (multiple myeloma, low-grade non-Hodgkin’s lymphoma, chronic lymphocytic leukemia).Citation64 The results showed that epoetin beta given weekly was as effective as the three times per week regimen (response rates of 72% versus 75%, respectively). Of note, 90% of patients treated with 30,000 IU epoetin beta once weekly remained transfusion-free throughout the study.Citation64
Another randomized trial comparing these two epoetin beta dose regimens (10,000 IU three times weekly versus 30,000 IU once weekly) in 100 patients with head and neck cancer and hemoglobin <10 g/dL demonstrated improvement in hemoglobin levels, energy levels, and quality of life to a similar extent on both regimens, along with comparable 2-year overall survival.Citation65 In addition, the efficacy of epoetin beta 30,000 IU once weekly was demonstrated in an observational, prospective study by Pirker et al.Citation66 They evaluated the response to epoetin beta of anemic (hemoglobin <12 g/dL) lung cancer patients (n=40, 72.5% with non-small cell lung cancer and 27.5% with small cell lung cancer) receiving chemotherapy. By the second chemotherapy cycle, the majority of patients (72.5%) required administration of epoetin beta, which induced a mean increase of 1.3 g/dL by week 4. Of note, 95% of patients did not require RBC transfusions during the study.Citation66 Analogously, the efficacy of the same dose of epoetin beta was assessed by the multicenter, open-label, prospective NAUTICA trial in patients with solid and non-myeloid hematological malignancies undergoing chemotherapy.Citation67 Considering the intention-to-treat population (n=691), epoetin beta induced a hemoglobin response in 60.4% of all patients and in 61.2% of those with baseline hemoglobin <11 g/dL. Of note, no difference in hemoglobin response was observed between patients with solid tumors and those with non-myeloid malignancies (60.5% versus 60.2%).Citation67
Clinical safety
In recent years, research has focused mainly on the safety of epoetin beta, due to the conflicting data concerning the adverse effects and/or reduced survival in ESA-treated cancer patients undergoing chemotherapy. The multicenter, double-blind, randomized, placebo-controlled ENHANCE trial was conducted in 351 patients with head and neck cancer undergoing radiotherapy and receiving placebo (n=171) or epoetin beta 300 IU/kg three weekly (n=180).Citation68 Results from the intention-to-treat population showed that anemia was corrected in individuals receiving epoetin beta versus placebo (82% versus 15%), yet the former group experienced increased locoregional progression (relative risk [RR] 1.69; 95% confidence interval [CI] 1.16–2.47; P=0.007) and decreased survival (RR 1.39; 95% CI 1.05–1.84; P=0.02). Also, mortality due to cardiac and general disorders was higher among patients given epoetin beta versus those on placebo (ten versus five patients and nine versus no patients, respectively). The authors hypothesized that erythropoietin might impair disease control in patients undergoing radiotherapy.
Interestingly, in a subsequent study, samples from 154 patients enrolled in the above trial were analyzed by immunohistochemistry for expression of EpoR;Citation69 the results revealed that patients positive for EpoR and treated with epoetin beta had poorer locoregional progression-free survival compared with those given placebo (adjusted RR 2.07; 95% CI 1.27–3.36; P<0.01), whereas epoetin had no effect on the outcome in EpoR-negative patients (adjusted RR 0.94; 95% CI 0.47–1.90; P=0.86). Further studies provided evidence for the expression of erythropoietin and EpoR by head and neck cancer cells, a role of erythropoietin in mediating cell invasion in vitro,Citation70 and the correlation between EpoR expression and aggressive tumor behavior, as well as a dismal prognosis.Citation71 Of note, the per protocol analysis indicated no significant effect of epoetin beta on disease progression.Citation68
BRAVE, a multicenter, open-label, randomized controlled study, investigated the effect of epoetin beta therapy on survival in patients with metastatic breast cancer and hemoglobin levels <12.9 g/dL who were undergoing chemotherapy.Citation72 The results showed no effect of epoetin beta on overall survival (hazard ratio [HR] 1.07; 95% CI 0.87–1.33; P=0.522) or progression-free survival (HR 1.07; 95% CI 0.89–1.30; P=0.448). Compared to control, epoetin beta induced an increase of median hemoglobin levels by 1.6 g/dL at 24 weeks, but also more thromboembolic events (13% versus 6%; P=0.012), despite a similar serious thromboembolic event rate was observed between the two groups (4% versus 3%).Citation72
Finally, Fujisaka et al conducted the first randomized, placebo-controlled Phase III trial to assess the efficacy and safety of epoetin beta dosed according to the updated guidelines recommending treatment initiation when hemoglobin is <10 g/dL and without reaching levels >12 g/dL.Citation73 The study involved 186 CIA patients with lung or gynecological cancer and confirmed the efficacy of epoetin beta in reducing RBC transfusions (4.5% versus 19.6%; P=0.002). No significant differences in quality of life, adverse events, and 1-year overall survival were observed between the epoetin beta and placebo groups.Citation73 However, as the study was not powered to assess mortality, further studies are warranted.
Meta-analyses and systematic reviews
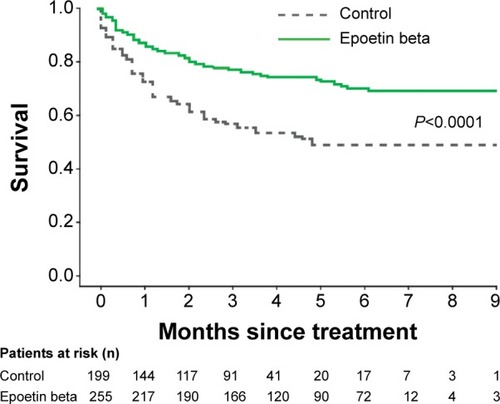
Various meta-analyses and systematic reviews have evaluated the efficacy and safety of epoetin beta in CIA patients. The study by Boogaerts et al included results from three randomized controlled trials conducted in patients with solid tumors receiving different chemotherapy regimens (with or without platinum) and treated with epoetin beta (n=255) or standard of care (n=199).Citation74 Upon administration of epoetin beta, a rapid increase in hemoglobin level and reduced transfusion requirement were observed. Transfusion-free survival was improved in the epoetin beta group (), with no risk for tumor progression or overall survival.Citation74 The frequency and types of adverse events as well as the frequency of thromboembolic events were also similar between the groups (5.9% versus 4.5%).Citation74 Another study based on 12 randomized controlled studies (n=2,297 patients, 65% with solid tumors, and 35% with non-myeloid hematological malignancies) showed no significant effect of epoetin beta on overall survival of all patients (HR 1.13; 95% CI 0.87–1.46; P=0.355) and of those with baseline hemoglobin <11 g/dL (HR 1.09; 95% CI 0.80–1.47; P=0.579).Citation75 Although treated and untreated individuals had a similar risk of progression, a trend for a reduction of such risk was seen overall (HR 0.85; 95% CI 0.72–1.01; P=0.072) and in patients with hemoglo-bin <11 g/dL (HR 0.80; 95% CI 0.65–0.99; P=0.041). On epoetin beta, the time to thromboembolic events was shorter (HR 1.62; 95% CI 1.13–2.31; P=0.008) and their frequency higher (7% and 4%, respectively, P=0.008). However, the frequency of thromboembolic event-related deaths was only 1% in both treated and control patients.Citation75
Figure 3 Kaplan–Meier curve showing transfusion-free survival in all patients.
Following the updated recommendations for the use of ESAs only in patients with hemoglobin levels <10 g/dL to be kept <12 g/dL, Aapro et al performed a subanalysis and stratified patients by baseline hemoglobin. Results from this analysis showed that epoetin beta had no negative effect on survival or disease progression when therapy was initiated with hemoglobin levels ≤10 g/dL. On the contrary, when treatment was started with hemoglobin >11 g/dL, a negative effect on survival was observed. For all hemoglobin levels, a higher risk of thromboembolic events was reported in epoetin beta-treated patients.Citation76
The Cochrane Collaboration experts recently published a systematic review on the use of erythropoietin and darbepoetin in cancer patients, which comprised the results from 91 randomized controlled trials, representing a total of 20,102 patients.Citation77 This study confirmed the efficacy of ESAs in treating anemia. Indeed, on ESAs, patients had a decreased risk of RBC transfusions (RR 0.65; 95% CI 0.62–0.68; 70 trials, n=16,093), and more frequently experienced a hematological response (RR 3.93; 95% CI 3.10–3.71; 31 trials, n=6,413) and improved quality of life. However, during the active study period, ESAs increased mortality (HR 1.17; 95% CI 1.06–1.29; 70 trials, n=15,935) and decreased overall survival (HR 1.05; 95% CI 1.00–1.11; 78 trials, n=19,003). Also, the risk for thromboembolic events was higher in the treated group (RR 1.52; 95% CI 1.3–1.74; 57 trials, n=15,498), compared with controls.Citation77 More recently, a meta-analysis of three randomized placebo-controlled trials was carried out, representing 511 Japanese patients with solid tumors or lymphoma given ESAs (epoetin beta or darbepoetin alfa; n=273) or placebo (n=238).Citation78 Notably, ESAs reduced the risk of transfusion (RR 0.47; 95% CI 0.29–0.76), while having no significant impact on overall survival (unadjusted HR 1.00; 95% CI 0.75–1.34), mortality rate (2.6% and 2.9% of deaths reported in ESA-treated and placebo-treated groups, respectively), or frequency of thromboembolic events (0.7% and 1.7%, upon ESAs and placebo, respectively).Citation78 Also, following a prespecified subgroup analysis (ie, ESA-treated patients at 3 months), only a tendency for the lowest risk of mortality was found for patients with hemoglobin of 11–11.5 g/dL, whereas no effect of the highest hemoglobin level achieved on ESAs was observed.Citation78
Conclusion
CIA affects the vast majority of cancer patients. Since low hemoglobin levels in CIA patients have been shown to be associated with low quality of life and short survival, it is important not to under evaluate the presence of anemia in patients with cancer. Epoetin beta has proven efficacy in raising hemoglobin levels and reducing the requirement for RBC transfusions in CIA patients. However, uncertainties remain regarding its safety profile. Data from preclinical studies suggest that, at least in certain tumor types, ESAs may favor tumor proliferation, metastatization, and drug resistance by activating pathways targeted by current anticancer therapies. Some clinical trials have also reported a worse outcome in ESA-treated patients undergoing chemotherapy. Indeed, further preclinical studies exploring in depth the mechanisms of action of erythropoietin in cancer cells are warranted, along with long-term, large, randomized, placebo-controlled trials with clearly defined endpoints and data on survival, in order to clarify the involvement of epoetin beta in survival and disease progression. Meanwhile, in light of the increased risk for thromboembolic events during treatment with ESAs, epoetin beta should be used according to the current guidelines for treatment of CIA, as approved by regulatory authorities.
Disclosure
CR and CGE received fees for manuscript preparation on behalf of Primula Multimedia SRL. LG has no conflicts of interest to disclose. The writing of this manuscript was funded by Roche SpA, Italy.
References
- GroopmanJEItriLMChemotherapy-induced anemia in adults: incidence and treatmentJ Natl Cancer Inst199991191616163410511589
- LudwigHVan BelleSBarrett-LeePThe European Cancer Anaemia Survey (ECAS): a large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patientsEur J Cancer200440152293230615454256
- RodgersGM3rdBeckerPSBlinderMCancer- and chemotherapy-induced anemiaJ Natl Compr Canc Netw201210562865322570293
- WoodPAHrusheskyWJCisplatin-associated anemia: an erythropoietin deficiency syndromeJ Clin Invest1995954165016597706473
- BohliusJWeingartOTrelleSEngertACancer-related anemia and recombinant human erythropoietin – an updated overviewNat Clin Pract Oncol20063315216416520805
- SpivakJLThe anaemia of cancer: death by a thousand cutsNat Rev Cancer20055754355515965494
- MillerCBJonesRJPiantadosiSAbeloffMDSpivakJLDecreased erythropoietin response in patients with the anemia of cancerN Engl J Med199032224168916922342534
- LudwigHStrasserKSymptomatology of anemiaSemin Oncol2001282 Suppl 871411395846
- VogelzangNJBreitbartWCellaDPatient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue CoalitionSemin Hematol1997343 Suppl 24129253778
- CurtGABreitbartWCellaDImpact of cancer-related fatigue on the lives of patients: new findings from the Fatigue CoalitionOncologist20005535336011040270
- HowellDKeller-OlamanSOliverTKA pan-Canadian practice guideline and algorithm: screening, assessment, and supportive care of adults with cancer-related fatigueCurr Oncol2013203e233e24623737693
- CellaDLaiJ-SChangC-HPetermanASlavinMFatigue in cancer patients compared with fatigue in the general United States populationCancer200294252853811900238
- Van BelleSJ-PCocquytVImpact of haemoglobin levels on the outcome of cancers treated with chemotherapyCrit Rev Oncol Hematol200347111112853095
- BlohmerJ-UDunstJHarrisonLCancer-related anemia: biological findings, clinical implications and impact on quality of lifeOncology200568Suppl 1122115855812
- CaroJJSalasMWardAGossGAnemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative reviewCancer200191122214222111413508
- NismanBBarakVHubertAPrognostic factors for survival in metastatic breast cancer during first-line paclitaxel chemotherapyAnticancer Res2003232C1939194212820483
- HauserCAStocklerMRTattersallMHNPrognostic factors in patients with recently diagnosed incurable cancer: a systematic reviewSupport Care Cancer20061410999101116708213
- WatersJSO’BrienMERAshleySManagement of anemia in patients receiving chemotherapyJ Clin Oncol200220260160311786593
- Di MaioMPisanoCTambaroRThe prognostic role of pre-chemotherapy hemoglobin level in patients with ovarian cancerFront Biosci2006111585159016368539
- HasencleverDDiehlVA prognostic score for advanced Hodgkin’s disease. International Prognostic Factors Project on Advanced Hodgkin’s DiseaseN Engl J Med199833921150615149819449
- KleinHGSpahnDRCarsonJLRed blood cell transfusion in clinical practiceLancet2007370958541542617679019
- EgrieJCStricklandTWLaneJCharacterization and biological effects of recombinant human erythropoietinImmunobiology19861723–52132243542810
- EgrieJCDwyerEBrowneJKHitzALykosMADarbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietinExp Hematol200331429029912691916
- LittlewoodTJBajettaENortierJWVercammenERapoportBEpoetin Alfa Study GroupEffects of epoetin alfa on hematologic parameters and quality of life in cancer patients receiving nonplati-num chemotherapy: results of a randomized, double-blind, placebo-controlled trialJ Clin Oncol200119112865287411387359
- GabriloveJLCleelandCSLivingstonRBSarokhanBWinerEEinhornLHClinical evaluation of once-weekly dosing of epoetin alfa in chemotherapy patients: improvements in hemoglobin and quality of life are similar to three-times-weekly dosingJ Clin Oncol200119112875288211387360
- OsterborgABrandbergYMolostovaVRandomized, double-blind, placebo-controlled trial of recombinant human erythropoietin, epoetin beta, in hematologic malignanciesJ Clin Oncol200220102486249412011126
- BoogaertsMCoiffierBKainzCEpoetin Beta QOL Working GroupImpact of epoetin beta on quality of life in patients with malignant diseaseBr J Cancer200388798899512671693
- MäenpääJPuistolaURiskaHImpact of epoetin-beta on anemia and health-related quality of life in cancer patients: a prospective observational study using the generic 15D instrumentAnticancer Res20143452325232924778039
- VansteenkisteJPirkerRMassutiBDouble-blind, placebo-controlled, randomized phase III trial of darbepoetin alfa in lung cancer patients receiving chemotherapyJ Natl Cancer Inst200294161211122012189224
- BennettCLSilverSMDjulbegovicBVenous thromboembolism and mortality associated with recombinant erythropoietin and darbepoetin administration for the treatment of cancer-associated anemiaJAMA2008299891492418314434
- BohliusJSchmidlinKBrillantCRecombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trialsLancet200937396741532154219410717
- TonelliMHemmelgarnBReimanTBenefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysisCMAJ200918011E62E7119407261
- LudwigHCrawfordJOsterborgAPooled analysis of individual patient-level data from all randomized, double-blind, placebo-controlled trials of darbepoetin alfa in the treatment of patients with chemotherapy-induced anemiaJ Clin Oncol200927172838284719380447
- GlaspyJCrawfordJVansteenkisteJErythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomesBr J Cancer2010102230131520051958
- EngertAJostingAHaverkampHEpoetin alfa in patients with advanced-stage Hodgkin’s lymphoma: results of the randomized placebo-controlled GHSG HD15EPO trialJ Clin Oncol201028132239224520368566
- MoebusVJackischCLueckH-JIntense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III studyJ Clin Oncol201028172874288020458045
- UntchMFaschingPAKonecnyGEPREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel ± darbepoetin alfa in primary breast cancer – results at the time of surgeryAnn Oncol20112291988199821385882
- AaproMEmerging topics in anaemia and cancerAnn Oncol201223Suppl 10x289x29322987979
- RizzoJDBrouwersMHurleyPSeidenfeldJSomerfieldMRTeminSAmerican Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancerJ Oncol Pract20106631732021358963
- CanonJ-LVansteenkisteJBodokyGRandomized, double-blind, active-controlled trial of every-3-week darbepoetin alfa for the treatment of chemotherapy-induced anemiaJ Natl Cancer Inst200698427328416478746
- 20030125 Study Group TrialGlaspyJVadhan-RajSPatelRRandomized comparison of every-2-week darbepoetin alfa and weekly epoetin alfa for the treatment of chemotherapy-induced anemia: the 20030125 Study Group TrialJ Clin Oncol200624152290229716710026
- MeansRTJrKrantzSBProgress in understanding the pathogenesis of the anemia of chronic diseaseBlood1992807163916471391934
- GangatNWolanskyjAPAnemia of chronic diseaseSemin Hematol201350323223823953340
- European Medicines AgencyNeorecormon Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000116/human_med_000921.jsp&mid=WC0b01ac058001d124Accessed February 1, 2015
- RizzoJDSomerfieldMRHagertyKLUse of epoetin and darbepoetin in patients with cancer: 2007 American Society of Clinical Oncology/American Society of Hematology clinical practice guideline updateJ Clin Oncol200826113214917954713
- JacobsKShoemakerCRudersdorfRIsolation and characterization of genomic and cDNA clones of human erythropoietinNature198531360058068103838366
- RecnyMAScobleHAKimYStructural characterization of natural human urinary and recombinant DNA-derived erythropoietin. Identification of desarginine 166 erythropoietinJ Biol Chem19872623517156171633680293
- JelkmannWPhysiology and pharmacology of erythropoietinTransfus Med Hemother201340530230924273483
- DavisJMArakawaTStricklandTWYphantisDACharacterization of recombinant human erythropoietin produced in Chinese hamster ovary cellsBiochemistry1987269263326383607040
- LudwigHEpoetin beta in oncology: examining the current evidenceFuture Oncol200621213816556069
- AcsGAcsPBeckwithSMErythropoietin and erythropoietin receptor expression in human cancerCancer Res20016193561356511325818
- SzenajchJWcisloGJeongJ-YSzczylikCFeldmanLThe role of erythropoietin and its receptor in growth, survival and therapeutic response of human tumor cells. From clinic to bench – a critical reviewBiochim Biophys Acta201018061829520406667
- OkazakiTEbiharaSAsadaMYamandaSNiuKAraiHErythropoietin promotes the growth of tumors lacking its receptor and decreases survival of tumor-bearing mice by enhancing angiogenesisNeoplasia200810993293918714393
- RibattiDNicoBPerraMTErythropoietin is involved in angiogenesis in human primary melanomaInt J Exp Pathol201091649549920804540
- NicoBAnneseTGuidolinDFinatoNCrivellatoERibattiDEpo is involved in angiogenesis in human gliomaJ Neurooncol20111021515820614229
- HedleyBDChuJEOrmondDGRecombinant human erythropoietin in combination with chemotherapy increases breast cancer metastasis in preclinical mouse modelsClin Cancer Res201117196151616221856770
- HedleyBDAllanALXenocostasAThe role of erythropoietin and erythropoiesis-stimulating agents in tumor progressionClin Cancer Res201117206373638021750199
- TodaroMTurdoABartucciMErythropoietin activates cell survival pathways in breast cancer stem-like cells to protect them from chemotherapyCancer Res201373216393640024008319
- ZhouBDamrauerJSBaileySTErythropoietin promotes breast tumorigenesis through tumor-initiating cell self-renewalJ Clin Invest2014124255356324435044
- LiangKQiuSLuYFanZAutocrine/paracrine erythropoietin regulates migration and invasion potential and the stemness of human breast cancer cellsCancer Biol Ther2014151899824100272
- JensenJDMadsenJKJensenLWPedersenEBReduced production, absorption, and elimination of erythropoietin in uremia compared with healthy volunteersJ Am Soc Nephrol1994521771857993997
- FlahartyKKCaroJErslevAPharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy menClin Pharmacol Ther19904755575642188770
- ChapelSVeng-PedersenPHohlRJSchmidtRLMcGuireEMWidnessJAChanges in erythropoietin pharmacokinetics following busulfan-induced bone marrow ablation in sheep: evidence for bone marrow as a major erythropoietin elimination pathwayJ Pharmacol Exp Ther2001298282082411454947
- CazzolaMBeguinYKloczkoJSpickaICoiffierBOnce-weekly epoetin beta is highly effective in treating anaemic patients with lymphoproliferative malignancy and defective endogenous erythropoietin productionBr J Haematol2003122338639312877665
- GuptaSSinghPKBhattMLBClinical benefits of two different dosing schedules of recombinant human erythropoietin in anemic patients with advanced head and neck cancerBiosci Trends20104526727221068481
- PirkerRLehnertMMinarWOnce-weekly epoetin beta (30,000 IU) in anemic patients with lung cancer receiving chemotherapyLung Cancer2007551899417084484
- SpaëthDDesablensBRodonPEpoetin beta once-weekly therapy in anemic patients with solid tumors and non-myeloid hematological malignancies receiving chemotherapyOncology2008741–211211818547966
- HenkeMLaszigRRübeCErythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trialLancet200336293921255126014575968
- HenkeMMatternDPepeMDo erythropoietin receptors on cancer cells explain unexpected clinical findings?J Clin Oncol200624294708471317028293
- LaiSYChildsEEXiSErythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinomaOncogene200524274442444915856028
- LinY-TChuangH-CChenC-HClinical significance of erythropoietin receptor expression in oral squamous cell carcinomaBMC Cancer20121219422639817
- AaproMLeonardRCBarnadasAEffect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) studyJ Clin Oncol200826459259818235117
- FujisakaYSugiyamaTSaitoHRandomised, phase III trial of epoetin-β to treat chemotherapy-induced anaemia according to the EU regulationBr J Cancer201110591267127221959870
- BoogaertsMOberhoffCTen Bokkel HuininkWNowrousianMRHaywardCRBurgerHUEpoetin beta (NeoRecormon) therapy in patients with solid tumours receiving platinum and non-platinum chemotherapy: a meta-analysisAnticancer Res2006261B47948416739308
- AaproMScherhagABurgerHUEffect of treatment with epoetin-beta on survival, tumour progression and thromboembolic events in patients with cancer: an updated meta-analysis of 12 randomised controlled studies including 2301 patientsBr J Cancer2008991142218542079
- AaproMOsterwalderBScherhagABurgerHUEpoetin-beta treatment in patients with cancer chemotherapy-induced anaemia: the impact of initial haemoglobin and target haemoglobin levels on survival, tumour progression and thromboembolic eventsBr J Cancer2009101121961197119997109
- ToniaTMettlerARobertNErythropoietin or darbepoetin for patients with cancerCochrane Database Syst Rev201212CD00340723235597
- OhashiYUemuraYFujisakaYMeta-analysis of epoetin beta and darbepoetin alfa treatment for chemotherapy-induced anemia and mortality: individual patient data from Japanese randomized, placebo-controlled trialsCancer Sci2013104448148523331490
- Van CutsemETwelvesCCassidyJOral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: results of a large phase III studyJ Clin Oncol200119214097410611689577
- DouillardJYCunninghamDRothADIrinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trialLancet200035592091041104710744089
- AndréTLouvetCMaindrault-GoebelFCPT-11 (irinotecan) addition to bimonthly, high-dose leucovorin and bolus and continuous-infusion 5-fluorouracil (FOLFIRI) for pretreated metastatic colorectal cancer. GERCOREur J Cancer19993591343134710658525
- De GramontAFigerASeymourMLeucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancerJ Clin Oncol200018162938294710944126
- HurwitzHIFehrenbacherLHainsworthJDBevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancerJ Clin Oncol200523153502350815908660
- KabbinavarFHurwitzHIFehrenbacherLPhase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancerJ Clin Oncol2003211606512506171
- CunninghamDHumbletYSienaSCetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancerN Engl J Med2004351433734515269313
- PfeifferPSørbyeHEhrssonHShort-time infusion of oxaliplatin in combination with capecitabine (XELOX30) as second-line therapy in patients with advanced colorectal cancer after failure to irinotecan and 5-fluorouracilAnn Oncol200617225225816291583
- KimTWKangWKChangHMMulticenter phase II study of oral capecitabine plus irinotecan as first-line chemotherapy in advanced colorectal cancer: a Korean Cancer Study Group trialActa Oncol200544323023516076694
- Von PawelJSchillerJHShepherdFATopotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancerJ Clin Oncol199917265866710080612
- NodaKNishiwakiYKawaharaMIrinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancerN Engl J Med20023462859111784874
- HattangadiSMWongPZhangLFlygareJLodishHFFrom stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modificationsBlood2011118246258626821998215
