Abstract
Background/aim
In light of the controversial published literature, this study aims to examine the potential prognostic role of AR immunohistochemical expression in triple negative breast cancer (TNBC).
Patients and methods
Ninety patients with TNBC were included in this study; the associations between AR expression (Allred score), clinicopathological variables (stage, grade, histological subtype, tumor size, nodal status, age at diagnosis, Ki67 expression, and p53 expression), and overall survival were evaluated.
Results
AR expression was not associated with stage, grade, histological subtype, tumor size, nodal status, age at diagnosis, Ki67 expression, and p53 expression. AR immunopositivity was not associated with overall survival either at the univariate or at the multivariate Cox regression analysis (multivariate hazard ratio =0.66, 95% confidence interval: 0.26–1.70, P=0.393).
Conclusion
AR expression does not seem to play a prognostic role in TNBC.
Introduction
Triple negative breast cancer (TNBC) is an entity that is characterized by ER, PR, and HER2 negativity. TNBC is characterized by poor prognosis,Citation1,Citation2 as well as more frequent metastases in lungs and brain;Citation3,Citation4 chemotherapy represents the available effective modality of systemic therapy for this type of breast cancer.
Immunohistochemical studies have shown that ARs are expressed in approximately 70%–90% of invasive breast cancer;Citation5–Citation8 nevertheless, their role in the pathogenesis of breast cancer remains to be fully elucidated, together with the potential biochemical mechanisms. It has been suggested that androgens may mediate a multifaceted role; they may induce a growth-inhibitory response in breast cancer cell lines expressing ER, PR, and AR, such as T-47-D and ZR-75-1, whereas growth-inducing effects have been noted in the ER(+) and PR(+) MCF-7 cells.Citation9 On the other hand, regarding breast cancer cell lines that do not express ER and PR, but are still AR positive, androgens may exhibit a proliferative effect; therefore interactions with cofactors and co-repressors have been suggested as meaningful for the net effects of androgens in breast cancer.Citation9 Concerning potential cofactors, Garay et al highlighted the interaction in the pathways between EGFR and AR on the level of MAPK pathway, as well as the dependence of AR signaling on p21.Citation10
A variety of studies have examined the role of ARs in terms of prognosis of breast cancer, with controversial findings. Kuenen-Boumeester et al showed that AR expression was associated with better disease free survival at the univariate analysis but the association dissipated at the multivariate approach;Citation11 on the other hand, the Nurses Health Study suggested a non-significant association between AR status and breast cancer death.Citation5 AR positivity has been found correlated with negative lymph node metastasis,Citation12 smaller tumor size,Citation6,Citation12 low histological grade,Citation6,Citation12 no elevated preoperative serum cancer antigen 15-3 levels,Citation6 and p53-negativity.Citation12
The controversy is also apparent among the studies examining AR expression especially in TNBC. Mrklic et al suggested that AR expression correlated inversely with higher clinical stage, histological grade mitotic score and proliferation index measured by Ki-67, but not with disease free survival or overall survival.Citation13 On the contrary, the nested analysis within the Nurses Health Study suggested that women with AR-positive TNBC had a sizable and statistically significant (by 83%) increase in overall mortality compared with those with AR-negative tumors.Citation5
In light of the controversial results, this study aims to examine the potential prognostic role of AR immunohistochemical expression in TNBC. This question seems of special importance, given that a prognostic role for ARs would imply an opportunity for a targeted treatment in this group of breast cancers, whose treatment options are limited.
Patients and methods
Study sample
Patients were recruited during a 9-year period (2002–2010) in a tertiary reference center (Department of Clinical and Obstetrics, Alexandra General Hospital). Ninety consecutive cases of Caucasian women, with TNBC, were identified through a detailed database; a centralized histological review of all cases and controls has been performed for the ascertainment of the condition. Information on patients’ age, stage, grade, tumor size, nodal status, and histological subtype was obtained from patients’ charts; two independent pathologists evaluated the histopathological variables.
Written informed consent was obtained from all participants in this study. This study conforms with the Helsinki Declaration and has been approved by the local Institutional Review Board.
Immunohistochemistry
To ensure the triple negative nature of the breast cancer cases, PgR (636, Dako Denmark A/S, Glostrup, Denmark), ER (ID5, Dako) and HER2 (CB11, Novocastra™) antibodies were used. Sections (thickness: 4 µm) were cut from formalin-fixed paraffin embedded tissues. The breast carcinoma tissue sections were deparaffinized in xylene and hydrated in graded ethanol solutions; thereafter, they were pretreated to enhance antigen retrieval Primary antibodies against PgR, ER, and HER2 were used in the EnVision + System-HRP (DAB) DakoCytomation (Dako Denmark A/S). Immunohistochemistry was conducted following the manufacturer’s instructions.
AR immunohistochemistry was performed at the Institute of Cancer Research, Medical University of Vienna using a standard protocol. Specifically, tissue sections were deparaffinized and rehydrated. Slides were incubated in 0.3% H2O2 for 10 minutes to reduce nonspecific background staining. Subsequently, specimens were heated for 10 minutes in 1 mM ethylenediaminetetraacetic acid (EDTA) buffer (pH 8.0) in a pressure cooker for epitope retrieval. Specimens were incubated with Ultra V Block (UltraVision LP detection system; Lab Vision Corporation, Fremont, CA, USA) at room temperature for 5 minutes to block background staining, incubation for 60 minutes at room temperature with a mouse monoclonal antibody specific for AR (AR441, Dako) followed. To detect antibody binding, the UltraVision LP detection system was used, following the manufacturer’s instructions. Color development with 3-3′-diaminobenzidine was performed and counterstaining with hematoxylin was conducted. Breast cancer sections known to express AR were used as positive controls.
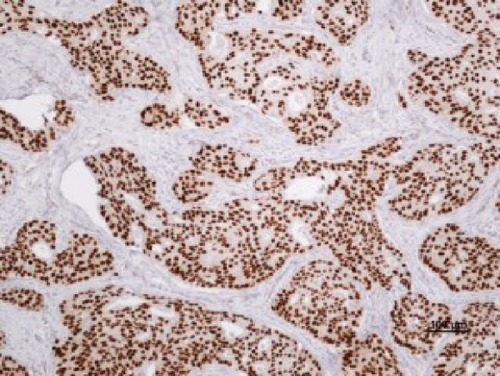
The examination of AR expression was assessed in a blinded manner regarding the patients’ clinical data. At least 100 tumor cells were evaluated for each patient. Immunostaining was quantified on the basis of nuclear staining intensity as well as percentage of AR-positive tumor cells using the Allred score. portrays a case with high AR expression.
Statistical methods
For the evaluation of the association between overall survival and expression of ARs, a standard two-step approach was followed: univariate and multivariate survival analysis; univariate and multivariate Cox regression analysis was undertaken, respectively. An alternative approach, with the AR Allred score treated as a continuous variable, was also followed so as to explore the reproducibility of any findings.
Apart from the overall analysis a subgroup analysis in the most prevalent histological subtype, namely ductal TNBC, was performed. Statistical analysis was performed using STATA/SE 13 statistical software (StataCorp LP, College Station, TX, USA).
Results
Descriptive statistics of the study sample are presented in , by AR expression status. AR expression was not associated with stage (P=0.444), grade (P=0.696), histological subtype (P>0.999), size of tumor (P=0.087), nodal status (P=0.080), age at diagnosis (P=0.105), Ki67 expression (P=0.698), and p53 expression (P=0.735). Anthracycline and taxane-based chemotherapy was received in 48.9% of patients, 24.4% received anthracycline-based chemotherapy, 12.2% taxane-based chemotherapy, 6.7% CMF regimen, whereas 7.8% did not receive chemotherapy. In our series no cases presented with metastases; therefore, a relevant analysis is not presented.
Table 1 Descriptive statistics of the study sample
The subgroup analysis in ductal TNBC (n=67) replicated the lack of associations; AR was not associated with stage (P=0.391), grade (P=0.092), tumor size (P=0.195), nodal status (P=0.119), age at diagnosis (P=0.091), Ki67 expression (P=0.393), and p53 expression (P=0.853).
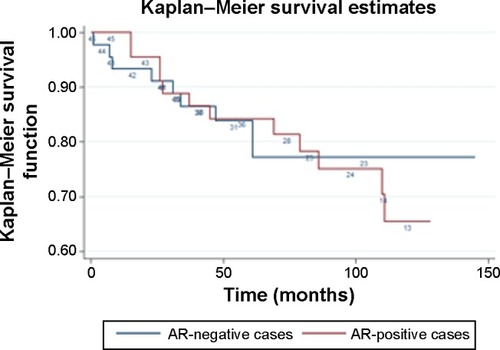
The median follow-up (defined as the median follow-up among those patients who did not die) was 87 months; the 5-year overall survival was 84.0%. At the univariate survival analysis, AR expression was not associated with overall survival either when it was treated as a binary variable (univariate hazard ratio [HR] =1.13, 95% confidence interval [CI]: 0.47–2.69, P=0.784 for AR positivity versus negativity) or as a continuous variable (univariate HR =0.99, 95% CI: 0.85–1.14, P=0.871 for 1-point increase in the All-red score) in the total TNBC sample. presents the Kaplan–Meier overall survival curves for AR-positive and AR-negative cases.
The results of the multivariate Cox regression analysis are presented in . Apart from an association between poorer survival and more advanced stage (multivariate HR =2.82, 95% CI: 1.33–5.99, P=0.007 for one level increase), no associations implicating grade, age at diagnosis, and AR immunopositivity (multivariate HR =0.66, 95% CI: 0.26–1.70, P=0.393, versus AR negativity) were detected. An alternative model with AR Allred score treated as continuous variable replicated the lack of association with overall survival (multivariate HR =0.89, 95% CI: 0.75–1.04, P=0.136 for 1-point increase in the Allred score).
Table 2 Results of multivariate Cox regression analysis for overall survival
The subgroup analysis on ductal carcinomas similarly yielded non-significant associations in both approaches to AR expression (multivariate HR =0.73, 95% CI: 0.24–2.28, P=0.593 for the binary approach and multivariate HR =0.91, 95% CI: 0.76–1.09, P=0.289 for the continuous approach).
Discussion
The main findings of this study pertain to the lack of a significant prognostic effect mediated by AR immunohistochemical expression. AR positivity was not associated either with the compendium of clinicopathological findings (grade, stage, histological type, tumor size, nodal status, Ki67 expression, and p53 expression) or with overall survival; the null associations were replicated in the subgroup of ductal carcinomas.
The published literature has yielded extremely controversial findings regarding the potential prognostic role of AR expression in TNBC. Similarly to our findings, Mrklic et al supported that AR expression was not associated with disease free survival or overall survival; nevertheless, their study pointed to an indirect prognostic role, as AR expression correlated inversely with higher mitotic score, clinical stage, histological grade, and Ki-67 proliferation index.Citation13 A similar pattern was noted in the study by Rakha et al where the multivariate analysis did not point to any independent prognostic effect mediated by AR expression in TNBC, despite its associations with grade, development of recurrences, and distant metastases.Citation14 In accordance with our findings, and adopting a more sophisticated methodology, namely reverse-phase protein arrays to measure AR levels, Gonzalez-Angulo et al supported that AR expression did not correlate with either overall or relapse-free survival in TNBC despite a borderline trend toward higher AR expression among cases with PIK3CA mutations.Citation15
In contrast with the null association detected in our sample, Luo et al supported a multifaceted favorable prognostic role of AR, as its expression was associated with tumor grade, nodal status, disease free survival, and overall survival in their series of TNBC.Citation16 Enlarging the perspective, Agoff et al examined ER-negative breast cancer cases, highlighting an association of AR expression with better disease free survival, lower grade, and smaller size.Citation17 On the other edge of the spectrum, AR expression was portrayed as an unfavorable prognostic factor in the nested analysis within the Nurses Health Study.Citation5
The AR positivity rate demonstrated in our TNBC series (equal to 50%) is compatible with the literature; Luo et al showed 27.7% positive immunostaining for AR,Citation16 Mrklic et al 32.5%,Citation13 Park et al 35%,Citation6 Ogawa et al 43%,Citation12 and Qi et al 53.2%.Citation7 Nevertheless, occasionally lower frequencies of AR expression among TNBC have been reported, with Rakha et al presenting 13% AR immunopositivity rate in their seriesCitation14 and Gonzalez-Angulo et al reporting 16.5% high AR levels via reverse-phase protein arrays.Citation15
Targeting AR with androgen-based hormonal therapy would represent an attractive treatment option for TNBC cases; nevertheless, our present, null results do not seem to justify AR-targeting therapy. The mechanisms underlying the present null findings remain elusive for the time being; ARs seem to exert pluripotent effects in breast cancer and interactions with cofactors and co-repressorsCitation9 may limit the net effects of their expression in TNBC; further subgrouping by the expression of potential cofactors may yield interesting results in future studies.
Despite the originality of this study, some limitations should be discussed and addressed. The quantification of AR expression was based on immunohistochemistry; more elaborate techniques, such as Western blot, would seem necessary for further validation of the present findings. At any case, the results of the alternative analysis treating AR expression as a continuous variable (Allred score) replicated the findings of the main analysis. Moreover, disease free survival was not available in our setting; therefore, the reproducibility of the overall survival-related findings upon disease free survival could not be examined. Furthermore, information regarding additional molecular indices associated with AR expression, such as PIK3CA mutations, was not available in our study.Citation15 Finally, our results should be further validated in larger studies, as our small sample size may have limited the statistical power in our analysis.
In conclusion, AR expression did not seem to exert a prognostic effect in our TNBC series, either in terms of clinicopathological variables or overall survival. Future studies adopting more elaborate techniques seem desirable to further validate the present findings and uncover the potential underlying mechanisms, cofactors, and molecular pathways.
Disclosure
The authors have no conflicts of interest to disclose.
References
- van de RijnMPerouCMTibshiraniRExpression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcomeAm J Pathol200216161991199612466114
- RouzierRPerouCMSymmansWFBreast cancer molecular subtypes respond differently to preoperative chemotherapyClin Cancer Res200511165678568516115903
- HicksDGShortSMPrescottNLBreast cancers with brain metastases are more likely to be estrogen receptor negative, express the basal cytokeratin CK5/6, and overexpress HER2 or EGFRAm J Surg Pathol20063091097110416931954
- FulfordLGReis-FilhoJSRyderKBasal-like grade III invasive ductal carcinoma of the breast: patterns of metastasis and long-term survivalBreast Cancer Res200791R417217540
- HuRDawoodSHolmesMDAndrogen receptor expression and breast cancer survival in postmenopausal womenClin Cancer Res20111771867187421325075
- ParkSKooJParkHSExpression of androgen receptors in primary breast cancerAnn Oncol201021348849219887463
- QiJPYangYLZhuHExpression of the androgen receptor and its correlation with molecular subtypes in 980 Chinese breast cancer patientsBreast Cancer (Auckl)201261822259247
- SoreideJALeaOAVarhaugJESkarsteinAKvinnslandSAndrogen receptors in operable breast cancer: relation to other steroid hormone receptors, correlations to prognostic factors and predictive value for effect of adjuvant tamoxifen treatmentEur J Surg Oncol19921821121181582503
- BirrellSNHallRETilleyWDRole of the androgen receptor in human breast cancerJ Mammary Gland Biol Neoplasia1998319510310819508
- GarayJPKarakasBAbukhdeirAMThe growth response to androgen receptor signaling in ERalpha-negative human breast cells is dependent on p21 and mediated by MAPK activationBreast Cancer Res2012141R2722321971
- Kuenen-BoumeesterVVan der KwastTHClaassenCCThe clinical significance of androgen receptors in breast cancer and their relation to histological and cell biological parametersEur J Cancer199632A9156015658911118
- OgawaYHaiEMatsumotoKAndrogen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkersInt J Clin Oncol200813543143518946753
- MrklicIPogorelicZCapkunVTomicSExpression of androgen receptors in triple negative breast carcinomasActa Histochem2013115434434823031358
- RakhaEAEl-SayedMEGreenARLeeAHRobertsonJFEllisIOPrognostic markers in triple-negative breast cancerCancer20071091253217146782
- Gonzalez-AnguloAMStemke-HaleKPallaSLAndrogen receptor levels and association with PIK3CA mutations and prognosis in breast cancerClin Cancer Res20091572472247819276248
- LuoXShiYXLiZMJiangWQExpression and clinical significance of androgen receptor in triple negative breast cancerChin J Cancer201029658559020507730
- AgoffSNSwansonPELindenHHawesSELawtonTJAndrogen receptor expression in estrogen receptor-negative breast cancer. Immu-nohistochemical, clinical, and prognostic associationsAm J Clin Pathol2003120572573114608899