Abstract
Background
The evidence for vitamin D reducing cancer risk is inconsistent, and it is not clear whether this reduction is related to variation in cytochrome P450 (CYP)24A1, the only enzyme known to degrade active vitamin D. We focused on evaluating the association of CYP24A1-rs2296241 polymorphism with hormone-related cancer risk by conducting a meta-analysis.
Methods
A systematic literature search was conducted in April 2014 (updated in December 2014) to identify eligible studies. A random-effects model was used to pool the odds ratio (OR).
Results
Eleven studies including 5,145 cases and 5,136 controls were considered for the allelic model, and eight studies of 3,959 cases and 3,560 controls were utilized for the additive, recessive, and dominant models. There was no significant association between CYP24A1-rs2296241 and hormone-related cancer risk in any of the models, yet substantial heterogeneity was observed. Subgroup analyses indicated that CYP24A1-rs2296241 variation reduced the prostate cancer risk in the additive (OR 0.91, 95% confidence interval 0.85–0.97) and recessive (OR 0.80, 95% confidence interval 0.67–0.95) models, with no evidence of heterogeneity.
Conclusion
This meta-analysis indicated that CYP24A1-rs2296241 polymorphism reduced the androgen-related prostate cancer risk in additive and recessive models. More genetic loci are needed to confirm the effect of CYP24A1 variation on the risk of prostate cancer.
Introduction
The scientific evidence linking vitamin D with carcinogenesis is increasing, but data suggesting that high levels of 25-hydroxyvitamin D3 [25(OH)D] reduce the risk of cancer are inconsistent. This could be because measured serum 25(OH)D levels may not correspond to vitamin D exposure or reflect tissue-specific levels. Before vitamin D can act as an anticancer agent, it must go through two steps of hydroxylation. Vitamin D is hydroxylated by 27-hydroxylase (cytochrome P450 [CYP]27A1 to 25(OH)D in the liver, followed by further metabolism by 1α-hydroxylase [CYP]27B1) to 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3).Citation1 The level of the main circulating form of vitamin D, 25(OH)D, is a widely accepted biomarker of vitamin D status, and 1,25(OH)2D3 is the activity of one that influence metabolic pathway, cell functions, and expression of target genes.Citation2 The final step in vitamin D metabolism is degradation of 25(OH)D and 1,25(OH)2D3 to 24,25(OH)D3 and 1,24,25(OH)2D3, respectively, which occurs via 25-hydroxyvitamin D 24-hydrolase (encoded by the CYP24A1 gene).Citation3 CYP24A1 has been clearly established as the main enzyme responsible for the degradation of active vitamin D. It is evident that CYP24A1 works in balance with CYP27B1. Elevated CYP24A1 expression and a reduced rate of CYP24A1 gene silencing has been reported in specific tumors, including the pancreas,Citation4 lung cancer,Citation5–Citation8 prostate cancer,Citation9 colon cancers,Citation10–Citation12 oral cancer,Citation13 and non-Hodgkin’s lymphoma.Citation14
CYP24A1 is located on 20q13.2 of chromosome 20; a number of polymorphisms of CYP24A1 have been identified, and the list is growing rapidly.Citation8,Citation9 Epidemiological studies have investigated the association between CYP24A1 variation and several hormone-related cancers, including thyroid carcinoma,Citation15 prostate cancer,Citation16–Citation19 and breast cancer.Citation20,Citation21 However, the results have been inconsistent, possibly because of small sample sizes, low statistical power, and clinical heterogeneity. In addition, there are overall 45 genetic loci of CYP24A1 in these hormone-related cancers, and 42 of them have reported only once. Locus rs2762941 has been studied in two articles, while rs927650 has been studied in five articles but two of them lacked original data. Only rs2296241 was found in all the studies, so we assessed the association between the CYP24A1-rs2296241 variant and hormone-related cancer risk by conducting a meta-analysis.
Methods
Study eligibility and selection
A systematic literature search was conducted in April 2014 (updated in December 2014) via PubMed, using the terms “CYP24A1”, “Vitamin D” or “25(OH)D” or “1,25(OH)2D3”, “cancer” or “carcinoma” as keywords. No restrictions were imposed. All eligible original studies, review articles, and other relevant studies were searched manually.
The criteria for inclusion were: study subjects were enrolled in case-control studies; the clinical outcome was hormone-related cancers; the exposure of interest was the CYP24A1-rs2296241 polymorphism; numbers of cases and controls with G/A, GG/GA, and GG/AA were provided, and genotype frequencies in the controls departed from Hardy–Weinberg equilibrium; and the odds ratio (OR) and corresponding 95% confidence interval (CI) were reported, or data to calculate them were included.
Data extraction
Two authors independently extracted data and collected the following: author, duration of follow-up, country, type of cancer, total number of cases and controls, and the number of cases and controls with G/A, GG/GA, and GG/AA for CYP24A1-rs2296241. The quality of each study was independently assessed by two authors using the Newcastle-Ottawa Scale.Citation22 The quality score was determined using three blocks of eight entries: selection of study population, contrast between case and control, and measurement of exposure factors. Total score ranged from 0 (worst) to 9 (best). A study was considered of high quality if the score was ≥5.
Statistical analysis
The meta-analysis was performed using allelic contrast (G versus A), recessive (GG versus GA+AA), dominant (GG+GA versus AA), and additive (GG versus AA) models. Point estimates of risk, and the OR and 95% CI were determined using a random-effects model. Hardy–Weinberg equilibrium was examined using a chi-square test. I2 was used to evaluate for statistical heterogeneity.Citation23,Citation24 Potential publication bias was assessed by examining funnel plots and using Begg’s test and Egger’s test.Citation25,Citation26 All statistical analyses were performed with STATA version 10.0 (Stata Corporation, College Station, TX) and SAS version 9.1 (SAS Institute, Cary, NC, USA).
Results
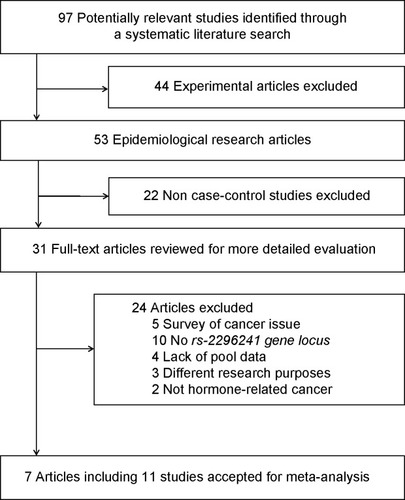
We identified 97 potentially relevant articles in our initial search. Of these, 44 papers on laboratory studies were excluded after the first screening based on the abstract or title, leaving 53 articles for full-text review. We excluded 22 articles for not being case-control studies. Among the remaining 31 articles, five were excluded for only the survey of cancer tissues, three for the observation of tumor recurrence and death, ten for lacking gene locus data, four for not having pooled data, and two for not including hormone-related cancers. Finally, seven publications representing eleven studies were included in the meta-analysis. A flow chart showing the study selection is presented in .
The characteristics of the eligible studies are shown in . The allelic contrast model included all eleven studies from the seven publications. In the other three models, six publications representing eight studies and three studies of prostate cancerCitation16 were excluded because they lacked detailed data for further evaluation. These studies were published between 2006 and 2014, and included two breast cancer,Citation20,Citation21 seven prostate cancer,Citation16–Citation19 and two thyroid carcinoma papers.Citation15 Two studies were conducted in Germany, six in the USA, two in Canada, and one in Korea.
Table 1 Characteristics of case-control studies included in the meta-analysis
The quality assessment showed that the quality scores ranged from 5 to 7 with a median score of 6, suggesting that all studies were of high quality (Table S1).
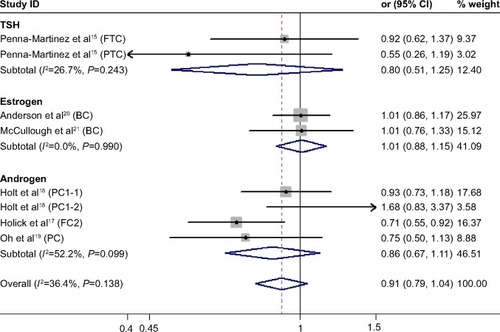
We found no significant association between CYP24A1-rs2296241 polymorphism and hormone-related cancer risk in any of the models (additive, OR 0.94, 95% CI 0.86–1.02; recessive, OR 0.86, 95% CI 0.72–1.01; allelic contrast, OR 1.03, 95% CI 0.96–1.11; dominant, OR 0.91, 95% CI 0.79–1.04; ). However, substantial heterogeneity was observed (I2>48.4%). Therefore, hormone-related cancers were further divided into thyroid cancer, breast cancer, and prostate cancer, and a sensitivity analysis was performed (Table S2).
Figure 2 Forest plot showing the association between CYP24A1-rs2296241 polymorphism and hormone-related cancer risk in the additive model.
Abbreviations: CI, confidence interval; CYP, cytochrome P450; TSH, thyroid-stimulating hormone; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
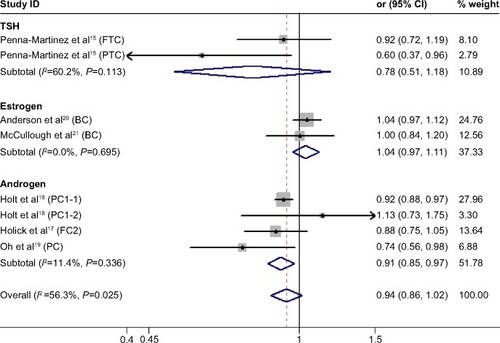
For thyroid cancer, including 353 cases and 305 controls, there was no statistically significance between the CYP24A1-rs2296241 polymorphism and cancer risk for any model (), and heterogeneity was still present in all models (all I2>50%). For breast cancer, including 2,277 cases and 2,339 controls, there was no association in any of the models (–), and no evidence of heterogeneity was observed (all I2=0%).
However, the CYP24A1-rs2296241 polymorphism significantly reduced the risk of prostate cancer, including 1,729 cases and 1,524 controls, in the additive (GG versus AA, OR 0.91, 95% CI 0.85–0.97, ) and recessive (GG versus GA+AA, OR 0.80, 95% CI 0.67–0.95, ) models, while no association was significantly observed in allelic contrast and dominant models ( and ). Heterogeneity was not observed in any model (all I2<25%), except for the dominant model (I2=52.2%).
Figure 3 Forest plot showing the association between CYP24A1-rs2296241 polymorphism and hormone-related cancer risk in the recessive mode.
Abbreviations: CI, confidence interval; CYP, cytochrome P450; TSH, thyroid-stimulating hormone; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
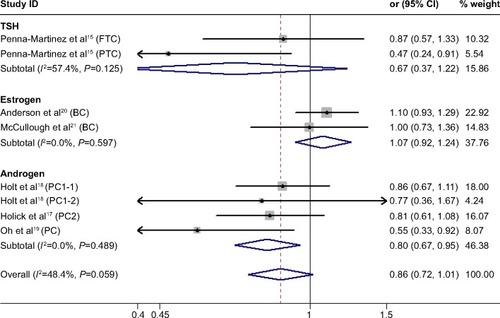
Figure 4 Forest plot showing the association between the CYP24A1-rs2296241 polymorphism and hormone-related cancer risk in the allelic contrast mode.
Abbreviations: CI, confidence interval; CYP, cytochrome P450; TSH, thyroid-stimulating hormone; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
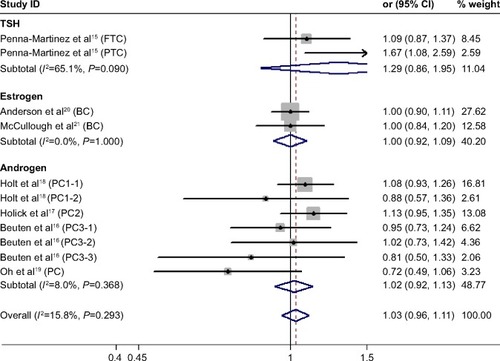
Figure 5 Forest plot showing the association between CYP24A1-rs2296241 polymorphism and hormone-related cancer risk in the dominant mode.
Abbreviations: CI, confidence interval; CYP, cytochrome P450; TSH, thyroid-stimulating hormone; FTC, follicular thyroid carcinoma; PTC, papillary thyroid carcinoma.
We did not find evidence of publication bias with either Begg’s test or Egger’s test in any of the models (all Begg’s tests P>0.05, all Egger’s tests P>0.05). Therefore, it is unlikely that publication bias had a significant influence on the observed association between CYP24A1-rs2296241 variation and reduced risk of prostate cancer in the additive model (GG versus AA) or recessive model (GG versus GA+AA).
Discussion
To our knowledge, until now, there has been no reported meta-analysis evaluating the association between CYP24A1 polymorphism and hormone-related cancer risk. In this meta-analysis, we found that variation in CYP24A1-rs2296241 was not significantly associated with the risk of hormone-related cancer in four models with evidence of substantial heterogeneity.
Substantial heterogeneity was not surprising, given the differences in tumor site and hormone type. Sensitivity analyses revealed a significant association between variation in CYP24A1-rs2296241 and a decreased risk of prostate cancer in the additive and recessive models. This hormonal influence is hardly surprising because the significance of androgens in the development of prostate cancer has been known for more than half century and androgens are major contributors to prostatic carcinogenesis.Citation27 Moreover, 1,25(OH)2D3, the main target degraded by CYP24A1 and expressed in most nucleated cells in local tissue, can also influence prostaglandin synthesis. Prostaglandin promotes carcinogenesis and facilitates cancer progression.Citation28 Even so, the mechanisms underlying the association between variation in CYP24A1 and the risk of cancer are unclear. CYP24A1-rs2296241 (conservation score 0.99) is a synonymous single nucleotide polymorphism (SNP) in exon 4. Although the SNP does not change the amino acid sequence, it could influence regulation and expression of the CYP24A1 gene and thereby alter protein levels. In addition, mutations in human CYP24A1 have been shown to modulate the regioselectivity of the enzyme.Citation29 Hence, CYP24A1 is deemed to be a 1,25(OH)2D3-binding protein first and a catabolic enzyme second.Citation30 This enzyme is rate-limiting for the amount of local vitamin D in cancer tissue, and elevated expression is more likely to lead to an adverse prognosis in cancer. Another explanation is that the true functional SNPs near the promoter region 5′ of exon 1 in CYP24A1, including rs2296241, rs4809960, rs2585428, and rs6022999, have a functional impact on vitamin D response element binding, and thereby could counteract the antitumorigenic effects of 1,25(OH)2D3.Citation11,Citation31,Citation32 Therefore, there are many claims that CYP24A1 has been identified as a proto-oncogene.Citation7,Citation8 In addition, serum androgen levels were negatively correlated with vitamin D status in men, and androgens are required for growth of prostate cancer in synergy with vitamin D receptor activity.Citation33 Therefore, CYP24A1-rs2296241 variation may be a reduced risk factor, meanwhile prolonged androgen stimulation may contribute to prostatic cancer.
Although there was no association between variation and thyroid cancer, there was significant heterogeneity in the four models. There was a small overlap in the CI for different thyroid-stimulating hormone-related cancers, and this could have affected the heterogeneity. Other than excess thyroid-stimulating hormone, pregnancies terminated by spontaneous or induced abortions can contribute to an increase in risk of thyroid cancer and significant heterogeneity.Citation27
We know that breast cancer is not only affected by estrogen and progesterone but also by age at menarche, menopause, and first full-term pregnancy.Citation27 Our subgroup analysis revealed no heterogeneity or association of risk between breast cancer and variation in CYP24A1-rs2296241, suggesting it is not a significant risk factor for breast cancer.
Several limitations in the current meta-analysis should be addressed. First, most studies had insufficient numbers, except for one,Citation20 which may have attenuated the statistical power, particularly for subgroup analysis. Second, because of the lack of original data, our results were based on unadjusted estimates of OR without adjustment for an individual’s disease history. Third, some heterogeneity existed that may be ascribed to roles of hormone and methods used for genotyping. Hence, we used the random-effects model that generated wider CIs for all genetic models. Finally, only one genetic locus of CYP24A1 variation was analyzed, because it appeared in all eleven studies.
The number of CYP24A1 polymorphisms in the genomic databases currently stands at around 50 and is growing rapidly. Two CYP24A1 polymorphisms (rs34043203 and rs2762934) have been associated with increased breast cancer risk and one (rs1570669) with reduced breast cancer risk.Citation35 There were a significant association of the CYP24A1-rs2296241 variant with a decreased risk of oral cancerCitation13 and head and neck cancer.Citation34 Significantly altered risks of recurrence/progression were observed in relation to genotype for two CYP24A1 SNPs (rs927650 and rs2762939), and five CYP24A1 SNPs (rs3787557, rs4809960, rs2296241, rs2585428, and rs6022999) significantly altered the risk of death from prostate cancer.Citation36 High levels of CYP24A1 are also found in prostate cancer cells.Citation10,Citation37 If overexpression of CYP24A1 directly affects tumor proliferation, tumor-targeted treatment with CYP24A1-specific inhibitors or mutagens may be effective in slowing tumor growth.
Conclusion
In summary, this meta-analysis indicates that variation in CYP24A1-rs2296241 may be associated with a reduced risk of androgen-related prostate cancer in additive (GG versus AA) and recessive (GG versus GA+AA) models. However, more genetic loci are needed to confirm the effect of CYP24A1 on the risk of androgen-related cancer.
Acknowledgments
This work was supported by grants from the National Natural Scientific Funding of China (81072286, 81372979), Jiangsu Natural Science Foundation (BK2012619), Chinese Nutrition Society Nutrition Research Foundation-DSM Research Fund, and Collaborative Innovation Center of Radiation Medicine, Jiangsu Higher Education Institution.
Supplementary materials
Table S1 Newcastle-Ottawa scale evaluation criteria of case-control study
Table S2 Meta-analysis of the association between CYP24A1-rs2296241 polymorphism and cancer risk
Disclosure
The authors report no conflicts of interest in this work.
References
- JonesGBiochemistry and metabolism of vitamin DRenzHTauberRAdvances in Clinical Chemistry and Laboratory MedicineBerlin, GermanyDeGruyter2012
- PikeJWGenome-wide principles of gene regulation by the vitamin D receptor and its activating ligandMol Cell Endocrinol201134731021664239
- JonesGProsserDEKaufmannM25-hydroxyvitamin D-24-hydroxylase (CYP24a1): its important role in the degradation of vitamin DArch Biochem Biophys201252391822100522
- AndersonLNCotterchioMKnightJABorgidaAGallingerSClearySPGenetic variants in vitamin D pathway genes and risk of pancreas cancer: results from a population-based case-control study in Ontario, CanadaPLoS One20138e6676823826131
- ChenGKimSHKingANCYP24a1 is an independent prognostic marker of survival in patients with lung adenocarcinomaClin Cancer Res20111781782621169243
- JohnsonCSChungITrumpDLEpigenetic silencing of CYP24 in the tumor microenvironmentJ Steroid Biochem Mol Biol201012133834220304059
- PariseRAEgorinMJKanterewiczBCYP24, the enzyme that catabolizes the antiproliferative agent vitamin D, is increased in lung cancerInt J Cancer20061191819182816708384
- AlbertsonDGYlstraBSegravesRQuantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogeneNat Genet20002514414610835626
- WhitlatchLWYoungMVSchwartzGG25-hydroxyvitamin D- 1α-hydroxylase activity is diminished in human prostate cancer cells and is enhanced by gene transferJ Steroid Biochem Mol Biol20028113514012137802
- HorváthHCLakatosPKósaJPThe candidate oncogene CYP24a1: a potential biomarker for colorectal tumorigenesisJ Histochem Cytochem20105827728519901270
- HöbausJHummelDMThiemUIncreased copy number and not DNA hypomethylation causes overexpression of the candidate proto – oncogene CYP24a1 in colorectal cancerInt J Cancer20131331380138823463632
- KosaJPHorvathPWolflingJCYP24a1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cellsWorld J Gastroenterol2013192621262823674869
- ZeljicKSupicGStamenkovicRMJovicNKozomaraRMagicZVitamin D receptor, CYP27b1 and CYP24a1 genes polymorphisms association with oral cancer risk and survivalJ Oral Pathol Med20124177978722612324
- KellyJLDrakeMTFredericksenZSEarly life sun exposure, vitamin D-related gene variants, and risk of non-Hodgkin’s lymphomaCancer Causes Control2012231017102922544453
- Penna-MartinezMRamos-LopezESternJImpaired vitamin D activation and association with CYP24a1 haplotypes in differentiated thyroid carcinomaThyroid20122270971622690899
- BeutenJGelfondJAFrankeJLSingle and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancerCancer Epidemiol Biomarkers Prev2009181869188019505920
- HolickCNStanfordJLKwonEMOstranderEANejentsevSPetersUComprehensive association analysis of the vitamin D pathway genes, VDR, CYP27b1, and CYP24a1, in prostate cancerCancer Epidemiol Biomarkers Prev2007161990199917932346
- HoltSKKwonEMPetersUOstranderEAStanfordJLVitamin D pathway gene variants and prostate cancer riskCancer Epidemiol Biomarkers Prev2009181929193319454612
- OhJJByunSSLeeSEGenetic variants in the CYP24a1 gene are associated with prostate cancer risk and aggressiveness in a Korean study populationProstate Cancer Prostatic Dis20141714915624492489
- AndersonLNCotterchioMColeDEKnightJAVitamin D-related genetic variants, interactions with vitamin D exposure, and breast cancer risk among Caucasian women in OntarioCancer Epidemiol Biomarkers Prev2011201708171721693626
- McCulloughMLStevensVLDiverWRVitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case-control studyBreast Cancer Res20079R917244366
- StangACritical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol20102560360520652370
- HigginsJPThompsonSGQuantifying heterogeneity in a meta-analysisStat Med2002211539155812111919
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ200332755756012958120
- BeggCBMazumdarMOperating characteristics of a rank correlation test for publication biasBiometrics199450108811017786990
- EggerMDavey SmithGSchneiderMMinderCBias in meta-analysis detected by a simple, graphical testBMJ19973156296349310563
- HendersonBEBernsteinLRossRKHormones and the etiology of cancerBastRCKufeDWPollockREHolland-Frei Cancer MedicineHamilton, ON, CanadaBC Decker2000
- FlanaganJNYoungMVPersonsKSVitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin DAnticancer Res2006262567257216886665
- MasudaSProsserDEGuoYDKaufmannMJonesGGeneration of a homology model for the human cytochrome p450, CYP24a1, and the testing of putative substrate binding residues by site-directed mutagenesis and enzyme activity studiesArch Biochem Biophys200746017719117224124
- AnnaloraAJBobrovnikov-MarjonESerdaRHybrid homology modeling and mutational analysis of cytochrome p450c24a1 (CYP24a1) of the vitamin D pathway: insights into substrate specificity and membrane bound structure-functionArch Biochem Biophys200746026227317207766
- OnenIHEkmekciAErogluMKonacEYesilSBiriHAssociation of genetic polymorphisms in vitamin D receptor gene and susceptibility to sporadic prostate cancerExp Biol Med200823316081614
- RoffAWilsonRTA novel SNP in a vitamin D response element of the CYP24a1 promoter reduces protein binding, transactivation, and gene expressionJ Steroid Biochem Mol Biol2008112475418824104
- InglesSARossRKYuMCAssociation of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptorJ Natl Cancer Inst1997891661708998186
- AzadAKBairatiIQiuXGenetic sequence variants in vitamin D metabolism pathway genes, serum vitamin D level and outcome in head and neck cancer patientsInt J Cancer20121322520252723169318
- FuhrmanBJFreedmanDMBhattiPSunlight, polymorphisms of vitamin D-related genes and risk of breast cancerAnticancer Res20133354355123393347
- HoltSKKwonEMKoopmeinersJSVitamin D pathway gene variants and prostate cancer prognosisProstate2010701448146020687218
- ChenTCHolickMFLokeshwarBLBurnsteinKLSchwartzGGEvaluation of vitamin D analogs as therapeutic agents for prostate cancerVitamin D Analogs in Cancer Prevention and TherapyNew York, NY, USASpringer2003