Abstract
Background
This study investigated the efficacy and safety of a new treatment strategy of combining panitumumab and bevacizumab, plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) versus FOLFIRI alone as second-line chemotherapy for metastatic colorectal cancer (mCRC) patients with known V-Ki-ras2 Kirsten rat sarcoma viral oncogene (KRAS) mutation status.
Methods
Patients with mCRC who had known KRAS tumor status and unsuccessful previous oxaliplatin-based chemotherapy were included in the study. They were randomly assigned to two groups to receive panitumumab and bevacizumab plus FOLFIRI, or FOLFIRI alone. In panitumumab and bevacizumab plus FOLFIRI group, patients were given 4 mg/kg panitumumab and bevacizumab plus FOLFIRI every 2 weeks.
Results
In all, 65 patients were assigned to panitumumab and bevacizumab plus FOLFIRI group, and 77 to FOLFIRI alone group. For WT KRAS patients, the median progression-free survival (PFS) was 5.7 months (95% confidence interval [CI], 2.4–7.5 months) for panitumumab and bevacizumab plus FOLFIRI and 3.8 months (95% CI, 3.0–6.7 months) for FOLFIRI alone; median overall survival (OS) was 15.2 months (95% CI, 8.9–19.7 months) for panitumumab and bevacizumab plus FOLFIRI and 11.0 months (95% CI, 8.2–15.4 months) for FOLFIRI alone. For MU KRAS patients, median PFS was 5.1 months (95% CI, 2.7–10.2 months) for panitumumab and bevacizumab plus FOLFIRI and 4.1 months (95% CI, 2.5–8.4 months) for FOLFIRI alone; median OS was 12.8 months (95% CI, 7.8–15.8 months) for panitumumab and bevacizumab plus FOLFIRI and 10.5 months (95% CI, 6.1–15.3 months) for FOLFIRI alone. Grade 3 and 4 adverse events were associated with panitumumab and bevacizumab plus FOLFIRI but tolerable among patients.
Conclusion
Patients with mCRC can be safely and efficiently treated with second-line chemotherapy of combining panitumumab and bevacizumab plus FOLFIRI, despite their KRAS mutation status.
Introduction
Colorectal cancer (CRC) is one of the three most common cancers in men, and one of the two most common cancers in women worldwide.Citation1 Every year, more than 1 million patients are diagnosed with CRC and most of them develop into metastatic colorectal cancer (mCRC).Citation1,Citation2 The standard single-agent chemotherapy for patients with mCRC typically uses regimen of 5-fluorouracil (5-FU) or leucovorin, oxali-platin and folinic acid, 5-FU and irinotecan (FOLFIRI).Citation3–Citation5 Recently, as we gained advanced knowledge on the underlying mechanisms of mCRC, the combination chemotherapy including targeted regimens, such as aflibercept, regorafenib, cetuximab, panitumumab, and bevacizumab, into traditional chemo-reagents for mCRC had shown a significant progress on extending patients’ long-term survival.Citation6–Citation10
The human homolog of V-Ki-ras2 Kirsten rat sarcoma viral oncogene (KRAS) is a GTPase protein that also acts as an oncogenic regulator. The mutation of KRAS is commonly found in various types of cancers, including mCRC.Citation11–Citation14 Studies demonstrated that KRAS mutation is highly associated with poor prognosis in patients with CRC.Citation13–Citation15 Panitumumab is an anti-epidermal growth factor receptor (EGFR) human monoclonal antibody, and has been applied in both first- and second-chemotherapy settings for patients with mCRC.Citation16–Citation20 However, clinical evidence showed mCRC patients with KRAS mutation, approximately 30%–50% of total patients with mCRC, responded poorly to panitumumab chemotherapy.Citation17,Citation18 Moreover, a recent randomized Phase III trial of second-line chemotherapy demonstrated that panitumumab plus FOLFIRI was only able to improve progression-free survival (PFS), but not overall survival (OS) in mCRC patients.Citation7
Bevacizumab is another human monoclonal antibody, thus has been utilized in target-specific chemotherapy for patients with mCRC. Unlike panitumumab, the target of bevacizumab is vascular endothelial growth factor (VEGF). It was suggested that KRAS protein might regulate the VEGF pathway to exert an angiogenic effect,Citation21,Citation22 and bevacizumab was shown to improve patients’ OS and response rates (RRs) in irinotecan-based chemotherapy of mCRC.Citation6,Citation23 However, it is not clear whether mCRC patients with mutated KRAS would directly benefit from bevacizumab chemotherapy.Citation24
Recently, two clinical studies demonstrated that combining panitumumab and bevacizumab plus FOLFIRI improved prognosis in patients with mCRC in second-line chemotherapy settings.Citation25,Citation26 However, these studies did not specify the relevance of KRAS mutation in combination chemotherapy. In the present study, we used a similar strategy of combining panitumumab and bevacizumab plus FOLFIRI as a second-line treatment option for patients with mCRC, and examined the patients’ response to treatment based on their KRAS mutation status.
Patients and methods
Patients
Eligible patients were between ≥18 and ≤85 years of age, having Eastern Cooperative Oncology Group (ECOG) performance status ≤2, diagnosed with metastatic adenocarcinoma of colon or rectum with at least one unidimensionally measurable lesion ≥20 mm based on magnetic resonance imaging (MRI) or computerized tomography (CT) imaging. Patients had to have only one prior oxaliplatin- or 5-FU-based chemotherapy but still diagnosed with disease progression within 6 months of first-line treatment. Patients had to have adequate hematologic, renal, and hepatic functions, and without any prior anti-VEGF or anti-EGFR treatment for mCRC.
Patients were excluded if they had received prior anti-EGFR or anti-VEGF therapy, had major surgery, hormonal therapy, immunotherapy within 4 weeks, or radiotherapy within 2 weeks of our study.
The protocol and treatment plan was approved by the Ethics Committee and Clinical Study Review Committee at Shandong Tumor Hospital. All patients provided signed consent forms before any procedures were conducted.
Study design and treatment schedule
This was an open-label, randomized, Phase II clinic study comparing the efficacy and safety of combining panitumumab and bevacizumab plus FOLFIRI. Patients were randomly stratified into two groups. In panitumumab and bevacizumab plus FOLFIRI group, patients were given 4 mg/kg panitumumab and 4 mg/kg bevacizumab plus FOLFIRI, every 2 weeks. Panitumumab and bevacizumab were administrated during a 90-minute infusion period before chemotherapy. In FOLFIRI alone group, patients were given FOLFIRI only (180 mg/m2 irinotecan, 400 mg/m2 racemic leucovorin by intravenous [IV] infusion on day 1 and FU 400 mg/m2 intravenous bolus on day 1, followed by 2,400 mg/m2 continuous infusion over days 1 and 2), every 2 weeks.
Responses were initially assessed by investigators, then confirmed by an independent radiologist blinded to the study, based on Response Evaluation Criteria in Solid Tumors (RECIST) every 8 weeks until disease progression. All patients were followed up on safety at least 45 days after the last study, and on survival every 90 days. During and after treatments, toxicity profiles were assessed for each patient. The adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.
KRAS determination
KRAS mutation was determined by an allele-specific PCR method according to the previous study.Citation27 Briefly, paraffin-embedded tumor tissues were deparaffinized, and DNA was extracted. A KRAS mutation kit (DXS, Farnham, UK) was used to detect seven mutated sites on codons 12 and 13, Gly12Asp, Gly12Ala, Gly12Val, Gly12Ser, Gly12Arg, Gly12Cys, and Gly13Asp. An experienced statistician at Core Clinical Laboratory then performed blinded KRAS analysis to validate the PCR results.
Statistical analysis
The primary endpoints were RR, OS, and PFS. Secondary endpoints were AEs. Statistical analysis was performed with a windows-based SPSS software (version 11.0). Sample size calculations were conducted with the assumption of one-sided 10% α, 80% power, and a 1:1 randomization. OS and PFS were estimated using the Kaplan–Meier model with a confidence interval (CI) of 95% for event-free probabilities and differences in probabilities, calculated by the Brookmeyer and Crowley method. Hazard ratios (HRs) were estimated by the Cox proportional hazards regression model.
Results
Patients
Between June 2010 and May 2014, there were a total of 142 patients who were eligible for the study. The CONSORT diagram is shown in . Among all eligible patients, 65 (46%) were assigned to panitumumab and bevacizumab plus FOLFIRI and 77 (54%) to FOLFIRI alone. Of those patients, 126 (89%) had test results of KRAS screening: 65 patients (52%) having wild-type (WT) KRAS tumors and 61 patients (48%) having mutant (MU) KRAS tumors.
Figure 1 CONSORT diagram for flow of patients through the study.
Baseline demographics and disease characteristics of the patients were stratified between those with WT and MU KRAS tumors, and balanced between two groups (panitumumab and bevacizumab plus FOLFIRI versus FOLFIRI alone), and balanced among (). For patients with WT KRAS tumors, the median ages were 59 years (21–82 years) in panitumumab and bevacizumab plus FOLFIRI group, and 62 years (25–80 years) in FOLFIRI alone group. For patients with MU KRAS tumors, the median ages were 61 years (22–79 years) in panitumumab and bevacizumab plus FOLFIRI group, and 60 years (29–85 years) in FOLFIRI alone group. For Eastern Cooperative Oncology Group performance status (ECOG PS), the majority of the patients had ECOG PS <2. For patients with WT KRAS tumors, 93% of those in panitumumab and bevacizumab plus FOLFIRI group, and 91% of those in FOLFIRI alone group, had ECOG PS of 0 and 1, respectively. For patients with MU KRAS tumors, 93% of those in panitumumab and bevacizumab plus FOLFIRI group, and 94% of those in FOLFIRI alone group, had ECOG PS of 0 and 1, respectively. In each group, for patients with either WT or MU KRAS tumors, about 60% of them had a primary tumor site in colon, and about 60% of them received first-line chemotherapy of FOLFOX.
Table 1 The demographics and disease characteristics of patients
Efficacy
PFSs and OSs were examined for patients with WT KRAS and MU KRAS tumors ().
Figure 2 Progression-free survival (A) or overall survival (B) for wild-type (WT) KRAS, and progression-free survival (C) or overall survival (D) for mutant (MU) KRAS were examined.
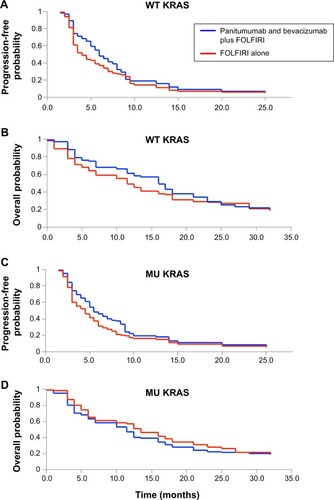
For patients with WT KRAS tumors, the median PFS was 5.7 months (95% CI, 2.4–7.5 months) for panitumumab and bevacizumab plus FOLFIRI and 3.8 months (95% CI, 3.0–6.7 months) for FOLFIRI alone. There was a significant improvement in PFS with panitumumab and bevacizumab plus FOLFIRI versus FOLFIRI alone (HR =0.45; 95% CI, 0.22–0.64; P<0.01, ). Median OS was 15.2 months (95% CI, 8.9–19.7 months) for panitumumab and bevacizumab plus FOLFIRI and 11.0 months (95% CI, 8.2–15.4 months) for FOLFIRI alone. There was also a significant improvement in OS with panitumumab and bevacizumab plus FOLFIRI versus FOLFIRI alone (HR =0.79; 95% CI, 0.47–0.89; P<0.01, ).
For patients with MU KRAS tumors, the median PFS was 5.1 months (95% CI, 2.7–10.2 months) for panitumumab and bevacizumab plus FOLFIRI and 4.1 months (95% CI, 2.5–8.4 months) for FOLFIRI alone. There was a significant improvement in PFS with panitumumab and bevacizumab plus FOLFIRI versus FOLFIRI alone (HR =0.65; 95% CI, 0.37–0.88; P<0.05, ). Median OS was 12.8 months (95% CI, 7.8–15.8 months) for panitumumab and bevacizumab plus FOLFIRI and 10.5 months (95% CI, 6.1–15.3 months) for FOLFIRI alone. There was also a significant improvement in OS with panitumumab and bevacizumab plus FOLFIRI versus FOLFIRI alone (HR =0.44; 95% CI, 0.19–0.61; P<0.05, ).
The RRs are shown in . For patients who had WT KRAS tumors, the objective response rate (OR) was 47% (95% CI, 32%–59%) in panitumumab and bevacizumab plus FOLFIRI group, significantly better than 26% (95% CI, 13%–41%) in FOLFIRI alone group (P<0.001). For patients who had MU KRAS, the OR was 44% (95% CI, 22%–53%) in panitumumab and bevacizumab plus FOLFIRI group, also significantly better than 29% (95% CI, 19%–55%) in the FOLFIRI alone group (P<0.05).
Table 2 Response rates of the patients
Safety
Grade 3 and 4 AEs for the patients with both WT and MU KRAS tumors are listed in . The incidence rates of grade 3 and 4 AEs in the WT KRAS patients in the panitumumab and bevacizumab plus FOLFIRI group and FOLFIRI alone group were 93% and 60%, respectively. The incidence rates of grade 3 and 4 AEs in the MU KRAS patients in the panitumumab and bevacizumab plus FOLFIRI group and FOLFIRI alone group were 93% and 56%, respectively.
Table 3 Grade 3 and 4 adverse events (AEs) of the patients
Moreover, despite KRAS tumor status, more patients experienced antibody-associated grade 3 and 4 AEs, including hypertension, bleeding, and proteinuria. For patients with WT KRAS tumors, the incidence rates of severe hypertension, bleeding, and proteinuria were 17%, 23%, and 13%, respectively, in panitumumab and bevacizumab plus FOLFIRI group. Those were higher than the rates of 6%, 3%, and 0%, in FOLFIRI alone group (P<0.01). For patients with MU KRAS tumors, the incidence rates of severe hypertension, bleeding, and proteinuria were 15%, 22%, and 19%, respectively, in panitumumab and bevacizumab plus FOLFIRI group, also significantly higher than the rates of 3%, 6%, and 3%, in FOLFIRI alone group (P<0.01).
Discussion
There have been two recent clinical studies, both in China, analyzing the treatment effects of combining anti-EGFR mAb and anti-VEGF mAb for patients with previously treated mCRC.Citation25,Citation26 However, neither of them correlated their results with KTAS mutation. Our study is the first to prospectively examine the efficacy and safety of combining panitumumab and bevacizumab according to the KRAS tumor status in patients with prior but failed first-line chemotherapy. And we demonstrated that, despite tumor status, panitumumab and bevacizumab plus FOLFIRI was effective in improving PFS and OS in patients with both WT and MU KRAS tumors.
In our study, for FOLFIRI alone treatment, the median PFSs were 3.8 months for patients with WT KRAS tumors and 4.1 months for patients with MU KRAS tumors; the median OSs were 11.0 months for patients with WT KRAS tumors and 10.5 months for patients with MU KRAS tumors; and ORs were 26% for patients with WT KRAS tumors and 29% for patients with MU KRAS tumors. These data were consistent with previous studies while using a similar second-line chemotherapy setting of biweekly treatment of FOLFIRI, reporting PFS ranging from 2 to 7 months, OS ranging from 8 to 15 months and disease-control rates ranging from 20% to 50%.Citation25,Citation28,Citation29 Thus, it suggests that, even the sample size of our study was smaller than previous ones, it was unlikely to confound the results of our study due to sampling errors.
Most importantly, we demonstrated that, despite the KRAS status, both WT and MU patients may benefit from the second-line chemotherapy combining panitumumab and bevacizumab. For patients with WT KRAS tumors, absolute improvements were observed in both PFS (5.7 months for panitumumab and bevacizumab plus FOLFIRI versus 3.8 months for FOLFIRI alone) and OS (15.2 months for panitumumab and bevacizumab plus FOLFIRI versus 11.0 months for FOLFIRI alone. Similar results were observed in MU KRAS patients, with absolute improvements in PFS (5.1 months versus 4.1 months) and OS (12.8 months versus 10.5 months), comparing panitumumab and bevacizumab plus FOLFIRI group and FOLFIRI alone group. This result is very encouraging as previous clinic trials showed that only those patients with WT KRAS tumors responded positively to panitumumab chemotherapy.Citation17,Citation18,Citation27 However, it was not clear, at least from current study, whether the combined regimen of bevacizumab was solely responsible for the survival improvements in patients with MU KRAS tumors, or adding bevacizumab into panitumumab resulted in synergetic effects on patients with MU KRAS tumors. Thus, future clinical studies comparing treatment effects between combination chemotherapy (panitumumab and bevacizumab) and mono-agent chemotherapy (panitumumab or bevacizumab) would help to optimize the treatment strategy of second-line chemotherapy for patients with mCRC.
Overall, our study clearly demonstrated that combination chemotherapy of panitumumab and bevacizumab plus FOLFIRI was effective in treating patients with mCRC in second-line setting with tolerable toxicity profiles. Patients with both WT and MU KRAS tumors can benefit from this combination chemotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- JemalASiegelRXuJWardECancer statistics, 2010CA Cancer J Clin20106027730020610543
- CiomborKKBerlinJTargeting metastatic colorectal cancer – present and emerging treatment optionsPharmgenomics Pers Med2014713714425045279
- CunninghamDPyrhonenSJamesRDRandomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancerLancet1998352141314189807987
- RougierPVan CutsemEBajettaERandomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancerLancet1998352140714129807986
- FuchsCSMarshallJMitchellERandomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C StudyJ Clin Oncol2007254779478617947725
- HurwitzHFehrenbacherLNovotnyWBevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancerNew Engl J Med20043502335234215175435
- PeetersMPriceTJCervantesARandomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancerJ Clin Oncol2010284706471320921462
- Van CutsemEKohneCHLangICetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation statusJ Clin Oncol2011292011201921502544
- MacarullaTSauriTTaberneroJEvaluation of aflibercept in the treatment of metastatic colorectal cancerExpert Opin Biol Ther2014141493150525152076
- SastreJArgilesGBenavidesMClinical management of regorafenib in the treatment of patients with advanced colorectal cancerClin Transl Oncol2014
- TimarJThe clinical relevance of KRAS gene mutation in non-small-cell lung cancerCurr Opin Oncol20142613814424463346
- BosJLRas oncogenes in human cancer: a reviewCancer Res198949468246892547513
- AndreyevHJNormanARCunninghamDKirsten ras mutations in patients with colorectal cancer: the ‘RASCAL II’ studyBr J Cancer20018569269611531254
- EstellerMGonzalezSRisquesRAK-ras and p16 aberrations confer poor prognosis in human colorectal cancerJ Clin Oncol20011929930411208819
- ImamuraYLochheadPYamauchiMAnalyses of clinicopathological, molecular, and prognostic associations of KRAS codon 61 and codon 146 mutations in colorectal cancer: cohort study and literature reviewMol Cancer20141313524885062
- CohnALShumakerGCKhandelwalPAn open-label, single-arm, phase 2 trial of panitumumab plus FOLFIRI as second-line therapy in patients with metastatic colorectal cancerClin Colorectal Cancer20111017117721855038
- MitchellEPPiperdiBLacoutureMEThe efficacy and safety of panitumumab administered concomitantly with FOLFIRI or Irinotecan in second-line therapy for metastatic colorectal cancer: the secondary analysis from STEPP (Skin Toxicity Evaluation Protocol With Panitumumab) by KRAS statusClin Colorectal Cancer20111033333922000810
- HockingCMPriceTJPanitumumab in the management of patients with KRAS wild-type metastatic colorectal cancerTherap Adv Gastroenterol201472037
- KohneCHHofheinzRMineurLFirst-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancerJ Cancer Res Clin Oncol2012138657221960318
- BerlinJPoseyJTchekmedyianSPanitumumab with irinotecan/leucovorin/5-fluorouracil for first-line treatment of metastatic colorectal cancerClin Colorectal Cancer2007642743217531105
- RakJYuJLKerbelRSCoomberBLWhat do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors?Cancer Res2002621931193411929804
- MizukamiYKohgoYChungDCHypoxia inducible factor-1 independent pathways in tumor angiogenesisClin Cancer Res2007135670567417908955
- HeinemannVHoffPMBevacizumab plus irinotecan-based regimens in the treatment of metastatic colorectal cancerOncology20107911812821088438
- HurwitzHIYiJInceWNovotnyWFRosenOThe clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancerOncologist200914222819144677
- XieSHanGFanZHeLXuWQinZSafety and efficacy of second-line treatment with folinic acid, 5-fluorouracil and irinotecan (FOLFIRI) in combination of panitumumab and bevacizumab for patients with metastatic colorectal cancerMed Oncol2014313524915899
- LiangHLHuAPLiSLLiuJYCombining bevacizumab and panitumumab with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as second-line treatment in patients with metastatic colorectal cancerMed Oncol20143197624793617
- AmadoRGWolfMPeetersMWild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancerJ Clin Oncol2008261626163418316791
- FuchsCSMooreMRHarkerGVillaLRinaldiDHechtJRPhase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancerJ Clin Oncol20032180781412610178
- MuroKBokuNShimadaYIrinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study)Lancet Oncol20101185386020708966
