Abstract
Introduction and objective
Many studies have been conducted on the association between the adenosine triphosphate-binding cassette, subfamily B, member 1 (ABCB1) gene C3435T polymorphism and leukemia risk, however, the previously published findings remain controversial. Thus, a meta-analysis was carried out to accurately evaluate the effect of this polymorphism on leukemia susceptibility.
Methods
A computerized literature search was conducted of PubMed, Elsevier database, the China National Knowledge Infrastructure database, and Wanfang Database, to find published case–control studies exploring the relationship between ABCB1 C3435T polymorphism and leukemia risk. Odds ratios (ORs) with 95% confidence intervals (CIs) were applied to assess the strength of association.
Results
A total of 17 studies of 2,431 cases and 3,028 controls were included in this meta-analysis. The results of overall comparisons suggest that there is a significant association between ABCB1 C3435T polymorphism and leukemia risk under two genetic models (TT vs CC: OR=1.39, 95% CI=1.04–1.84, P=0.02; CT+TT vs CC: OR=1.20, 95% CI=1.06–1.36, P=0.004). In the subgroup analyses by ethnicity, age, and leukemia subtype, a significant association was found in Caucasian (CT vs CC: OR=1.22, 95% CI=1.03–1.45, P=0.02; TT vs CC: OR=1.34, 95% CI=1.10–1.64, P=0.004; CT+TT vs CC: OR=1.27, 95% CI=1.08–1.49, P=0.004), adult leukemia (CT vs CC: OR=1.46, 95% CI=1.17–1.83, P=0.001; CT+TT vs CC: OR=1.43, 95% CI=1.01–2.03, P=0.04), and lymphocytic leukemia (TT vs CC: OR=1.73, 95% CI=1.19–2.51, P=0.004; TT vs CC+CT: OR=1.62, 95% CI=1.10–2.38, P=0.01; CT+TT vs CC: OR=1.28, 95% CI=1.10–1.48, P=0.001).
Conclusion
The meta-analysis suggests that ABCB1 C3435T polymorphism is associated with increased risk of leukemia.
Introduction
Leukemia is a group of malignant diseases of the hematopoietic system, with an estimated 54,270 new cases and 24,450 deaths expected in the USA in 2015.Citation1 Although the incidence of leukemia increases gradually, with age the most prominent risk factor, the etiology and mechanisms underlying the pathogenesis of leukemia are not yet fully understood.Citation2 Epidemiological studies have found that advanced age, radiation, smoking, and exposure to chemical carcinogens are risk factors which contribute to the genesis of leukemia.Citation3–Citation5 However, only a small proportion of people exposed to these risk factors develop leukemia, suggesting that host genetic factors might play an important role.Citation6 Recent genome-wide association studies have identified the presence of inherited genetic susceptibility to this disease.Citation7–Citation9 Therefore, multiple factors are considered implicated in the etiology of leukemia, including exogenous or endogenous exposure, genetic susceptibility, and chance.Citation2
The human adenosine triphosphate-binding cassette, subfamily B, member 1 (ABCB1) gene, also named multidrug-resistance 1 (MDR1) gene, is located at 7q21.1, with 28 exons encoding a 170 kDa membrane transporter called P-glycoprotein (P-gp). The presence of a highly conserved adenosine triphosphate (ATP)-binding site in two homologous halves as well as the linker region makes this protein a member of the adenosine triphosphate-binding cassette (ABC) superfamily.Citation10 P-gp acts as an efflux pump in an ATP-dependent fashion, transporting exogenous and endogenous substrates from the inside of cells to the outside. P-gp was first identified in human cancer cells as a protein responsible for resistance against many anticancer drugs. Subsequently, this efflux transporter has been found in various normal human tissues, including in the intestinal epithelium, adrenal gland, placenta, kidney, liver, capillary endothelial cells of the brain, and testes. Physiological expression of P-gp in excretory tissues provides a cellular defense mechanism against potentially harmful compounds.Citation10–Citation12 ABCB1 is polymorphic and at least 50 single-nucleotide polymorphisms (SNPs) have been identified within ABCB1 gene locus.Citation13 C3435T at exon 26 is the most widely investigated SNP of ABCB1 and has been associated with altered P-gp expression and activity in tissue studies including those on placenta, liver, and leukocytes.Citation14–Citation16 There is increasing evidence that genetic variants of ABCB1 affect P-gp activity and expression levels, and the alteration of P-gp transport activity results in decreased extrusion of harmful xenobiotics and cumulative cytotoxicity.Citation17,Citation18 The C3435T SNP in ABCB1 has been associated with the development of various cancers, including breast cancer, hepatocellular carcinoma, and non-Hodgkin lymphoma.Citation19–Citation21 Many case–control studies have been conducted to investigate whether the ABCB1 C3435T polymorphism is associated with leukemia risk but these have yielded controversial results. Therefore, we performed a meta-analysis to accurately evaluate the effect of ABCB1 C3435T polymorphism on leukemia susceptibility.
Materials and methods
Study identification
A systematic literature search of the PubMed, Elsevier, China National Knowledge Infrastructure, and Wanfang databases was conducted to identify studies that explored the relationship between ABCB1 C3435T polymorphism and leukemia risk. The search terms and keywords were as follows: “multidrug resistance gene” or “MDR1” or “ABCB1”, “polymorphism” or “variant” or “mutant”, and “leukemia” or “leukaemia” or “leukocythemia”. No language restriction was applied and the latest search was undertaken on April 30, 2014. The references cited in the eligible studies were also examined to find additional studies. Two reviewers examined the retrieved literature independently and disagreement was resolved by discussion.
Inclusion criteria
All studies included in this meta-analysis had to meet the following criteria. They had to: (1) be case–control studies assessing the relationship between ABCB1 C3435T polymorphism and leukemia risk; (2) have confirmed diagnosis in the case group; and (3) have genotype frequencies for both cases and controls available. Reviews, meta-analyses, case reports, and letters were excluded.
Data extraction
Two reviewers independently extracted information from each eligibility study and disagreements were addressed by discussion. The following data were extracted from each study: author(s), year of publication, country, ethnicity, source of controls, genotyping methods, sample size of cases and controls, genotype frequencies of the ABCB1 C3435T polymorphism for cases and controls, and Hardy–Weinberg equilibrium (HWE) of control group.
Statistical analysis
The HWE of the genotype distribution in the control groups was checked using the χ2 test and P-values <0.05 were designated as deviations from HWE. The strength of association between ABCB1 C3435T polymorphism and leukemia risk was assessed by odds ratios (ORs) with 95% confidence intervals (CIs) under the heterozygote model (CT vs CC), homozygote model (TT vs CC), dominant model (CT+TT vs CC), and recessive model (TT vs CC+CT). The significance of the combined OR was determined by the Z test. The Q-statistic test was used to evaluate the between-study heterogeneity. If P<0.05, indicating that there was significant heterogeneity, the random-effects model (DerSimonian–Laird) was applied to combine the data, otherwise, the fixed-effects model (Mantel–Haenszel) was selected. Subgroup analyses were performed by ethnicity, age, and leukemia subtype to find the source of heterogeneity. Funnel plots were produced to evaluate publication bias and sensitivity analysis was performed by omission of studies not in agreement with HWE to assess the stability of the results. All the tests were two-sided and P-values <0.05 were considered as statistically significant. The data analyses were performed using Review Manager software (v 5.2; The Cochrane Collaboration, Oxford, UK).
Results
Characteristics of studies
In accordance with the inclusion criteria, a total of 17 publications with 2,431 leukemia cases and 3,028 controls were included in the meta-analysis presented here.Citation22–Citation38 The main characteristics of included studies are summarized in . All of the 17 studies were case–control studies, 14 of which were population-based controls and the remaining three were hospital-based controls. All the cases had a confirmed diagnosis of leukemia and the controls were mainly matched for age and sex. Among the included studies, six studies were conducted in an Asian population,Citation25,Citation28,Citation31,Citation34,Citation36,Citation38 nine studies in a Caucasian population,Citation22–Citation24,Citation26,Citation29,Citation30,Citation32,Citation33,Citation35 and two studies in a mixed population.Citation27,Citation37 The distribution of genotypes among the control groups in four studies was not in agreement with HWE.Citation22,Citation33,Citation35,Citation36
Table 1 Main characteristics of studies included in the meta-analysis
Meta-analysis results
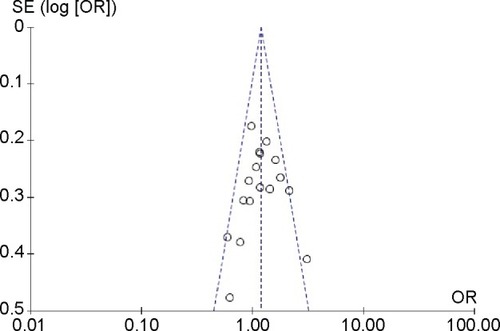
lists the main results of this meta-analysis. When all the eligible studies were pooled into the meta-analysis, a significant association between ABCB1 C3435T polymorphism and leukemia risk was observed under two genetic models (TT vs CC: OR=1.39, 95% CI=1.04–1.84, P=0.02; CT+TT vs CC: OR=1.20, 95% CI=1.06–1.36, P=0.004) (). Subgroup analyses were also performed to explore the effect of ethnicity, age, and leukemia subtype. When stratified by ethnicity, a significantly increased risk was observed in Caucasians (CT vs CC: OR=1.22, 95% CI=1.03–1.45, P=0.02; TT vs CC: OR=1.34, 95% CI=1.10–1.64, P=0.004; CT+TT vs CC: OR=1.27, 95% CI=1.08–1.49, P=0.004) (). Significant associations were also found in adult leukemia (CT vs CC: OR=1.46, 95% CI=1.17–1.83, P=0.001; CT+TT vs CC: OR=1.43, 95% CI=1.01–2.03, P=0.04) (, ) and lymphocytic leukemia (TT vs CC: OR=1.73, 95% CI=1.19–2.51, P=0.004; TT vs CC+CT: OR=1.62, 95% CI=1.10–2.38, P=0.01; CT+TT vs CC: OR=1.28, 95% CI=1.10–1.48, P=0.001) (, ).
Figure 1 Forest plot of association of adenosine triphosphate-binding cassette, subfamily B, member 1 (ABCB1) C3435T polymorphism with leukemia risk, subgroup analysis by age (CT vs CC).
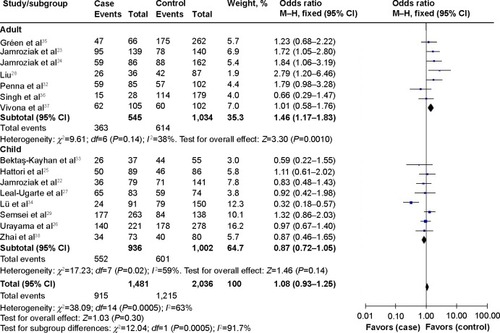
Figure 2 Forest plot of association of adenosine triphosphate-binding cassette, subfamily B, member 1 (ABCB1) C3435T polymorphism with leukemia risk, subgroup analysis by leukemia subtype (CT+TT vs CC).
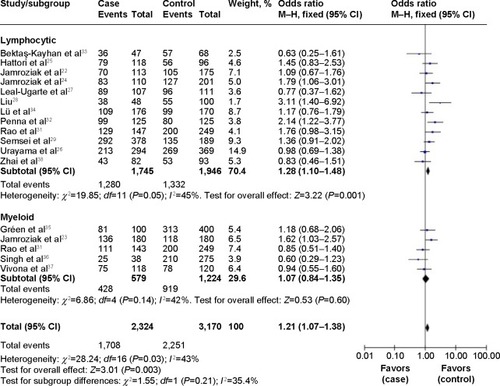
Table 2 Results of meta-analysis for adenosine triphosphate-binding cassette, subfamily B, member 1 (ABCB1) C3435T polymorphism and leukemia risk
Sensitivity analysis and publication bias
Sensitivity analysis was performed by excluding the studies in which the distribution of genotypes in the control groups was not in agreement with HWE. The results show that the statistical significance was not changed, suggesting that the results of this meta-analysis are relatively stable and credible. Four funnel plots were produced to assess the publication bias and the shapes of funnel plots reveal no evidence of obvious asymmetry, indicating there is no statistical evidence of publication bias ().
Discussion
At least 50 SNPs have been described in all 28 exons of the ABCB1 gene and the C3435T (rs1045642) polymorphism is a synonymous variant with no effect on amino-acid change at codon 1142 (Ile1142Ile) in the second ATP-binding domain.Citation13,Citation17 Studies have shown that 3435T is associated with lower ABCB1 expression levels and subsequently reduces P-gp activity and alters substrate specificity.Citation14,Citation39,Citation40 P-gp is not only expressed in tumor cells, but also in cells of several normal tissues. It was detected in the apical membranes of excretory tissues, such as the intestine, kidney, and liver, suggesting its role in the elimination of xenobiotics.Citation41,Citation42 Besides, P-gp is also expressed in peripheral leukocytes and hematopoietic stem cells from which acute myeloid leukemia originates, which indicates that ABCB1 may play an important role in the etiology of leukemia.Citation43,Citation44 Functional polymorphisms in ABCB1 may cause various human malignancies including leukemia. However, the exact biological mechanism for the association of ABCB1 polymorphisms with leukemia risk still requires explanation through further studies.
Given the potential influence of this functional polymorphism on cancer susceptibility, many molecular epidemiological studies have been conducted to investigate the association between ABCB1 C3435T polymorphism and leukemia risk. However, the results from different studies are controversial, which may be owing to different genetic backgrounds in individual studies. To synthetically evaluate the effect of ABCB1 C3435T polymorphism on susceptibility to leukemia, we performed a meta-analysis of 17 case–control studies that assessed the relationship between ABCB1 C3435T polymorphism and leukemia risk. In this meta-analysis, in overall comparisons under a homozygote model and dominant model, we found that the ABCB1 3435TT genotype significantly increases leukemia risk. When stratified by ethnicity, the results indicate that the individuals with a TT genotype in the C3435T of ABCB1 had significantly increased risk of leukemia among Caucasian populations, but not in other ethnicities. An explanation for this is that the racial differences in leukemia incidence can partly be attributed to differences in genotype frequencies between different populations at ABCB1 loci. In the subgroup analysis by age, we found significant association between ABCB1 C3435T polymorphism and increased risk in adult leukemia in the heterozygote model and dominant model, but not in children with leukemia under any comparison models. When restricting the analysis to the subtype of leukemia, significant association was found in lymphocytic leukemia in the homozygote model, recessive model, and dominant model, but not in myeloid leukemia in any comparison model, which indicates that clinical type might have a critical effect on the association.
The results of this study show that ABCB1 C3435T polymorphism might modify susceptibility to leukemia, which is consistent with the meta-analysis reported by Qian et al.Citation45 Since our study combined a total of 2,431 cases and 3,028 controls from 17 case–control studies, our results are more convincing.
Limitations
Some limitations of this meta-analysis should be taken into consideration and caution is needed when the results are interpreted. First, non-differential misclassification bias is possible, as the controls were not uniformly defined. Population-based controls and hospital-based controls have different risks of evolving leukemia. Second, our analyses were based on estimates not adjusted for other risk factors such as folate-intake status, lifestyle, and environmental exposures, which might influence the combined results. Third, although subgroup analyses were performed by ethnicity, age, and leukemia subtype to find the sources of heterogeneity, significant heterogeneity still existed in some subgroups. The heterogeneity may have been caused by different lifestyles, exposure to different risk factors, and the different levels of exposure to risk factors. In addition, due to the limited original data, potential gene–gene and gene–environment interactions which have an important impact on leukemia risk were not evaluated in this study.
Conclusion
This meta-analysis found that ABCB1 C3435T polymorphism is associated with an increased risk of leukemia and is likely a risk factor facilitating leukemia development. However, well-designed case–control studies with larger sample size focusing on more ethnicities or leukemia subtypes are required to validate our findings in the future.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- InabaHGreavesMMullighanCGAcute lymphoblastic leukaemiaLancet201338198811943195523523389
- FinchSCRadiation-induced leukemia: lessons from historyBest Pract Res Clin Haematol200720110911817336261
- BjörkJJohanssonBBrobergKAlbinMSmoking as a risk factor for myelodysplastic syndromes and acute myeloid leukemia and its relation to cytogenetic findings: a case-control studyLeuk Res200933678879119019430
- LichtmanMAObesity and the risk for a hematological malignancy: leukemia, lymphoma, or myelomaOncologist201015101083110120930095
- MullighanCGGenetic variation and the risk of acute lymphoblastic leukemiaLeuk Res201034101269127020538337
- SherborneALHoskingFJPrasadRBVariation in CDKN2A at 9p21.3 influences childhood acute lymphoblastic leukemia riskNat Genet201042649249420453839
- WangYChenJLiJAssociation of three polymorphisms in ARID5B, IKZF1 and CEBPE with the risk of childhood acute lymphoblastic leukemia in a Chinese populationGene2013524220320723608171
- SpeedyHEDi BernardoMCSavaGPA genome-wide association study identifies multiple susceptibility loci for chronic lymphocytic leukemiaNat Genet2014461566024292274
- SchinkelAHThe physiological function of drug-transporting P-glycoproteinsSemin Cancer Biol1997831611709441946
- SunJHeZGChengGWangSJHaoXHZouMJMultidrug resistance P-glycoprotein: crucial significance in drug disposition and interactionMed Sci Monit2004101RA5RA1414704647
- HodgesLMMarkovaSMChinnLWVery important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein)Pharmacogenet Genomics201121315216120216335
- BreierABarancíkMSulováZUhrikBP-glycoprotein – implications of metabolism of neoplastic cells and cancer therapyCurr Cancer Drug Targets20055645746816178819
- HoffmeyerSBurkOvon RichterOFunctional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivoProc Natl Acad Sci U S A20009773473347810716719
- KurataYIeiriIKimuraMRole of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoproteinClin Pharmacol Ther200272220921912189368
- LambaJStromSVenkataramananRMDR1 genotype is associated with hepatic cytochrome P450 3A4 basal and induction phenotypeClin Pharmacol Ther200679432533816580901
- MarzoliniCPausEBuclinTKimRBPolymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevanceClin Pharmacol Ther2004751133314749689
- Brambila-TapiaAJMDR1 (ABCB1) polymorphisms: functional effects and clinical implicationsRev Invest Clin201365544545424687344
- BaldisseraVDde MattosAACoralGPEvaluation of the C3435T polymorphism in the MDR1 gene in patients with hepatocellular carcinomaAnn Hepatol201211689990623109454
- WangZWangTBianJAssociation between MDR1 C3435T polymorphism and risk of breast cancerGene20135321949924070710
- KimHNKimNYYuLPolymorphisms in DNA repair genes and MDR1 and the risk for non-Hodgkin lymphomaInt J Mol Sci20141546703671624756092
- JamroziakKMłynarskiWBalcerczakEFunctional C3435T polymorphism of MDR1 gene: an impact on genetic susceptibility and clinical outcome of childhood acute lymphoblastic leukemiaEur J Haematol200472531432115059065
- JamroziakKBalcerczakECebulaBJanusAMirowskiMRobakTNo influence of 3435C>T ABCB1 (MDR1) gene polymorphism on risk of adult acute myeloid leukemia and P-glycoprotein expression in blast cellsTher Drug Monit200628570771117038891
- JamroziakKBalcerczakESmolewskiPMDR1 (ABCB1) gene polymorphism C3435T is associated with P-glycoprotein activity in B-cell chronic lymphocytic leukemiaPharmacol Rep200658572072817085864
- HattoriHSuminoeAWadaMRegulatory polymorphisms of multidrug resistance 1 (MDR1) gene are associated with the development of childhood acute lymphoblastic leukemiaLeuk Res200731121633164017568669
- UrayamaKYWienckeJKBufflerPAChokkalingamAPMetayerCWiemelsJLMDR1 gene variants, indoor insecticide exposure, and the risk of childhood acute lymphoblastic leukemiaCancer Epidemiol Biomarkers Prev20071661172117717548681
- Leal-UgarteEGutiérrez-AnguloMMacías-GómezNMMDR1 C3435T polymorphism in Mexican children with acute lymphoblastic leukemia and in healthy individualsHum Biol200880444945519317599
- LiuRRThe role of multidrug resistance gene 1 polymorphism to prognosis of adult acute lymphoblastic leukemia patientsCentral South University20085148
- SemseiAFErdélyiDJUngváriIAssociation of some rare haplotypes and genotype combinations in the MDR1 gene with childhood acute lymphoblastic leukaemiaLeuk Res20083281214122018243305
- RochaVPorcherRFernandesJFAssociation of drug metabolism gene polymorphisms with toxicities, graft-versus-host disease and survival after HLA-identical sibling hematopoietic stem cell transplantation for patients with leukemiaLeukemia200923354555619005482
- RaoDNAnuradhaCVishnupriyaSAssociation of an MDR1 gene (C3435T) polymorphism with acute leukemia in IndiaAsian Pac J Cancer Prev20101141063106621133625
- PennaGAllegraAAlonciAMDR-1 polymorphisms (G2677T and C3435T) in B-chronic lymphocytic leukemia: an impact on susceptibility and prognosisMed Oncol20112841549155420496015
- Bektaş-KayhanKKüçükhüseyinÖKaragözGIs the MDR1 C3435T polymorphism responsible for oral mucositis in children with acute lymphoblastic leukemia?Asian Pac J Cancer Prev201213105251525523244145
- LüHDuZZWangWRelationship between genetic polymorphism of multidrug resistance 1 gene and the risk of childhood acute lymphocytic leukemiaZhonghua Er Ke Za Zhi2012509692696 Chinese23158821
- GréenHFalkIJLotfiKAssociation of ABCB1 polymorphisms with survival and in vitro cytotoxicity in de novo acute myeloid leukemia with normal karyotypePharmacogenomics J201212211111820938465
- SinghOChanJYLinKHengCCChowbayBSLC22A1-ABCB1 haplotype profiles predict imatinib pharmacokinetics in Asian patients with chronic myeloid leukemiaPLoS One2012712e5177123272163
- VivonaDBuenoCTLimaLTABCB1 haplotype is associated with major molecular response in chronic myeloid leukemia patients treated with standard-dose of imatinibBlood Cells Mol Dis201248213213622134106
- ZhaiXWangHZhuXGene polymorphisms of ABC transporters are associated with clinical outcomes in children with acute lymphoblastic leukemiaArch Med Sci20128465967123056078
- SongPLambaJKZhangLG2677T and C3435T genotype and haplotype are associated with hepatic ABCB1 (MDR1) expressionJ Clin Pharmacol200646337337916490813
- Kimchi-SarfatyCOhJMKimIWA “silent” polymorphism in the MDR1 gene changes substrate specificityScience2007315581152552817185560
- TeraoTHisanagaESaiYTamaiITsujiAActive secretion of drugs from the small intestinal epithelium in rats by P-glycoprotein functioning as an absorption barrierJ Pharm Pharmacol19964810108310898953513
- TanigawaraYRole of P-glycoprotein in drug dispositionTher Drug Monit200022113714010688277
- ElliottJIRaguzSHigginsCFMultidrug transporter activity in lymphocytesBr J Pharmacol2004143789990715492020
- PawlikABaskiewicz-MasiukMMachalinskiBKurzawskiMGawronska-SzklarzBInvolvement of C3435T and G2677T multidrug resistance gene polymorphisms in release of cytokines from peripheral blood mononuclear cells treated with methotrexate and dexamethasoneEur J Pharmacol20055281–3273616321374
- QianXCaoSYangGVariant genotypes of MDR1 C3435T increase the risk of leukemia: evidence from 10 case-control studiesLeuk Lymphoma20125361183118722088099