Abstract
Background
People’s Republic of China is one of the countries with the highest incidence of gastric cancer, accounting for 45% of all new gastric cancer cases in the world. Therefore, strong prognostic markers are critical for the diagnosis and survival of Chinese patients suffering from gastric cancer. Recent studies have begun to unravel the mechanisms linking the host inflammatory response to tumor growth, invasion and metastasis in gastric cancers. Based on this relationship between inflammation and cancer progression, several inflammation-based scores have been demonstrated to have prognostic value in many types of malignant solid tumors.
Objective
To compare the prognostic value of inflammation-based prognostic scores and tumor node metastasis (TNM) stage in patients undergoing gastric cancer resection.
Methods
The inflammation-based prognostic scores were calculated for 207 patients with gastric cancer who underwent surgery. Glasgow prognostic score (GPS), neutrophil lymphocyte ratio (NLR), platelet lymphocyte ratio (PLR), prognostic nutritional index (PNI), and prognostic index (PI) were analyzed. Linear trend chi-square test, likelihood ratio chi-square test, and receiver operating characteristic were performed to compare the prognostic value of the selected scores and TNM stage.
Results
In univariate analysis, preoperative serum C-reactive protein (P<0.001), serum albumin (P<0.001), GPS (P<0.001), PLR (P=0.002), NLR (P<0.001), PI (P<0.001), PNI (P<0.001), and TNM stage (P<0.001) were significantly associated with both overall survival and disease-free survival of patients with gastric cancer. In multivariate analysis, GPS (P=0.024), NLR (P=0.012), PI (P=0.001), TNM stage (P<0.001), and degree of differentiation (P=0.002) were independent predictors of gastric cancer survival. GPS and TNM stage had a comparable prognostic value and higher linear trend chi-square value, likelihood ratio chi-square value, and larger area under the receiver operating characteristic curve as compared to other inflammation-based prognostic scores.
Conclusion
The present study indicates that preoperative GPS and TNM stage are robust predictors of gastric cancer survival as compared to NLR, PLR, PI, and PNI in patients undergoing tumor resection.
Introduction
Gastric cancer remains a leading cause of cancer mortality, despite a decline in incidence worldwide. According to the International Agency for Research on Cancer (IARC), one million new cases of gastric cancer are diagnosed each year. Each year, gastric cancer causes over 700,000 deaths worldwide.Citation1 People’s Republic of China is one of the countries with the highest incidence of gastric cancer, accounting for 45% of all new gastric cancer cases in the world. In People’s Republic of China, gastric cancer represents the third leading cause of cancer mortality, after lung and liver cancers.Citation2 Therefore, strong prognostic markers are critical for the diagnosis and survival of Chinese patients suffering from gastric cancer.
At present, surgery is the only proven effective treatment for gastric cancer. With earlier detection of gastric tumors, more and more patients benefit from initial diagnosis and improved surgical techniques. Currently, calculation of the tumor node metastasis (TNM) stage remains the major tool for clinical prognosis evaluation in gastric cancer. However, the accuracy of the TNM stage is limited due to individual differences in gastric cancer patients. Thus, there is an urgent need to establish optimal methods for surgical prognosis.
Recent studies have begun to unravel the mechanisms linking the host inflammatory response to tumor growth, invasion, and metastasis in gastric cancers.Citation3,Citation4 It is well known that the signaling networks required for tumor invasion and the influx of tumor-infiltrating lymphocytes produce various inflammatory cytokines and chemokines.Citation5,Citation6 These inflammatory cytokines, such as interleukin 6 (IL-6), in addition to tumor-associated inflammatory cells, such as neutrophils, contribute to tumor angiogenesis, invasion, and metastasis in the tumor, thereby worsening the prognosis of the patients.Citation7–Citation10 Additionally, it has been shown that blockade of inflammatory cytokines and chemokines greatly inhibits the progression of various tumors.Citation5 Based on this relationship between inflammation and cancer progression, several inflammation-based scores have been demonstrated to have prognostic value in many types of malignant solid tumors. The main scores include Glasgow prognostic score (GPS),Citation11–Citation18 neutrophil lymphocyte ratio (NLR),Citation19,Citation20 platelet lymphocyte ratio (PLR),Citation21,Citation22 prognostic nutritional index (PNI),Citation23,Citation24 and prognostic index (PI).Citation24,Citation25 These scores take into account the size, environment, and leukocyte ratio of the inflammatory lesion to create a predictive prognosis score. Previous studies have demonstrated that the GPS has superior prognostic value when compared to NLR, PLR, PI, and PNI in hepatocellular and other malignant cancers.Citation24,Citation26–Citation28 Additionally, a preoperative high NLR score is also associated with poor survival in patients with gastric cancer.Citation29–Citation32 While many groups have shown that GPS is an independent risk predictor in gastric cancer, no studies have been done to include GPS in addition to PI, PNI, and TNM stage for comparison purposes.Citation31,Citation32 This would allow us to determine combinations of inflammation-based scores and TNM stage that may have high predictive values. To our knowledge, there are no studies elucidating which of these prognostic scores is more suitable in predicting outcomes in patients with gastric cancer undergoing surgery.
Therefore, we performed a retrospective study to comprehensively compare the prognostic value of preoperative inflammation-based prognostic scores (GPS, NLR, PLR, PI, PNI) and TNM stage in terms of overall survival (OS) and disease-free survival (DFS) in a cohort of Chinese gastric cancer patients.
Materials and methods
Patients and samples
This retrospective study included 207 patients who underwent resection of gastric cancer between June 2005 and September 2011 at the First Affiliated Hospital of Fujian Medical University. All the surgeries were performed by the same team. All blood samples were collected before the operation and all the postoperative specimens were histologically confirmed. None of the patients had distant metastases, preoperative chemotherapy or radiotherapy, or clinical evidence of infection or other inflammatory conditions (eg, vasculitis, rheumatoid arthritis) prior to surgery. Patients whose deaths were caused by reasons other than malignant stomach tumors or gastric cancer were also excluded from the study. The TNM staging was according to the TNM classification criteria from seventh edition of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UIUC). Ethical approval was obtained from the Research Ethic Committee of First Hospital of Quanzhou; informed consent was obtained from each patient.
In the case of stage II, III, and IV gastric cancers, adjuvant chemotherapy was performed for 4–6 weeks after surgery. A combination of fluorouracil, platinum, and anthracyclines were used according to the National Comprehensive Cancer Network guidelines. The chemotherapy was given once every 3 weeks for a total of six cycles. Only patients who had received at least four cycles of chemotherapy were regarded as receiving adjuvant chemotherapy after surgery.
Patient follow-up
Follow-up was performed for all 207 patients every 1–3 months for the first 2 years, every 3–6 months for 2–5 years, and every 6 months after 5 years following surgery until September 1, 2014. The follow-up included reviewing the patient’s medical history, in addition to serum tumor marker measurement, B-scanning, and CT scanning. The period from the operation to the date of death was defined as the OS time. The recurrence of gastric cancer was defined as the occurrence of new lesions that were confirmed by imaging or histology examination. The period from the operation to the date of recurrence was defined as the DFS time. The median follow-up time was 37 months after operation and the interval ranged from 3 to 83 months.
Measurement of serum GPS, NLR, PLR, PNI, and PI
Prior to surgery, blood samples were collected and routine laboratory analyses of these prognostic markers were performed. The scores of GPS, NLR, PLR, PNI, and PI are given in .
Table 1 Systemic inflammation-based prognostic scores
Defining prognostic value in TNM staging system
The accuracy of a prognostic system was related to homogeneity (comparable survival rate among patients with the same TNM stage), discriminatory ability (significant survival differences among patients with different TNM stages), and monotonicity of gradients (worse survival rate in patients with more advanced TNM stages compared to earlier TNM stages).Citation33 Homogeneity within each TNM stage was determined by the likelihood ratio (LR). The discriminatory ability and monotonicity of gradients of the staging system were quantified using linear trend (LT) χ2 value as well as the area under the receiver operating characteristic (ROC) curve. Lastly, the independent contribution of each staging system to overall prediction of survival in the Cox model was evaluated by comparing the LR test in a reduced model when one stage system was removed and in the full model (all systems included). The higher the ratio, the higher prognostic value the corresponding staging system has.
Statistical analysis
All the statistical analyses were performed using the SPSS 19.0 software for Windows (SPSS Inc., Chicago, IL, USA). Data with heterogeneity of variance were analyzed by rank sum test. All other data, unless otherwise noted, were analyzed by student’s t-test. Comparison of OS and DFS rates were performed by χ2 test and Fisher’s exact probability. A one-way analysis of variance (ANOVA) was used to compare quantitative data among multiple groups. The postoperative survival rate was performed by Kaplan–Meier analysis and long rank tests to identify significance. Univariate and multivariate analyses were conducted using Cox proportional hazards model and the inspection level α=0.05. A comparison of categorical variables was performed by calculating the area under the ROC curve for 1-, 3-, and 5-year postoperative survival rates. Cases that lacked survival data in the respective time periods were excluded. Significance was defined as P<0.05.
Results
Patient characteristics
In our study, we report 140 (67.6%) males and 67 females (32.4%) with an average age of 64 years (standard deviation [SD] ±12). All patients suffered from gastric cancer and were in need of surgery. The postoperational histology study indicated 180 (87.0%) cases of gastric adenocarcinoma, 21 (10.1%) cases of mucinous adenocarcinoma, and 6 (2.9%) cases of signet ring cell carcinoma. Fifty-three (25.6%) patients had moderately to well-differentiated diffuse carcinoma, while 154 (73.4%) patients had poorly differentiated diffuse carcinoma. Therefore, our study shows a trend toward poorly differentiated diffuse carcinoma in our population. Proximal (cardia, fundus) carcinoma occurred in 92 (44.4%) cases, middle (gastric body) carcinoma in 49 (23.7%) cases, distal (antrum) carcinoma in 61 (29.5%) cases, multiple carcinomas in 3 (1.4%) cases, and remnant carcinoma in 2 (1.0%) cases. Based on the criteria of seventh edition of AJCC/UIUC, the following TNM stages were assigned: stage I, 16 patients (7.7%); stage II, 46 patients (22.2%); stage III, 136 patients (65.7%); and stage IV, 9 patients (4.3%). Therefore, the majority of patients enrolled in this study were stage III gastric cancer patients. The overall 1-, 3-, and 5-year survival rates after operation were 75.4%, 48.3%, and 27.5%, respectively, with an average survival time of 34 months. Five patients (2.5%) were lost to follow-up by the end of the survey on September 1, 2014. In most patients, gastric cancer returned after the initial surgery. One hundred and eight-five (91.6%) patients had recurrent gastric cancer, and the overall morbidity rate was 86.6% (175 patients). Patient data are detailed in .
Table 2 Univariate and multivariate analyses of clinicopathologic factors in relation to postoperative outcome for overall survival and disease-free survival
Correlation between prognostic scores, clinicopathological features, and OS rate
Univariate analysis demonstrated that TNM stage (P<0.001) (), GPS (P<0.001) (), preoperative weight loss (P=0.034), serum C-reactive protein (CRP) (P<0.001), serum albumin (P<0.001), NLR (P<0.001) (), PLR (P=0.002) (), PI (P<0.001) (), PNI (P<0.001) (), degree of differentiation (P<0.001), and adjuvant chemotherapy (P=0.006) were significantly associated with 3- and 5-year OS (). All inflammatory-based prognostic scores were associated with survival. Moreover, patients with preoperative weight loss over 5 kg, high initial CRP level, hypoalbuminemia, high initial inflammation-based prognostic sores (NLR, PLR, PI, PNI), high TNM stage, low differentiation, or patients who did not undergo adjuvant therapy had worse OS rate, indicating that initial screening as well as adjuvant therapy may be important for increased survival prognosis.
Figure 1 Kaplan–Meier survival curves showing the relationship between the information-based scores and overall survival in patients with (A) TNM, (B) GPS, (C) NLR, (D) PLR, (E) PI, and (F) PNI.
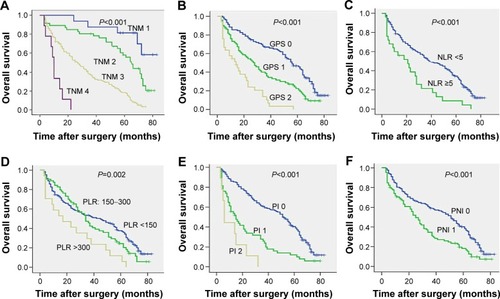
Next, we performed a COX risk model analysis for each of the significant prognostic markers identified by univariate analysis to demonstrate whether these markers could serve as independent risk factors in patients with gastric cancer. The result suggested that TNM stage (95% CI, 2.258–4.116; P<0.001), degree of differentiation (95% CI, 0.410–0.829; P=0.002), GPS (95% CI, 1.043–1.698; P=0.024), NLR (95% CI, 1.133–2.763; P=0.012), PI (95% CI, 1.198–2.102; P=0.001), and adjuvant chemotherapy (95% CI, 0.292–0.587; P<0.001) were all independent risk factors for postoperational OS time, while PLR and PNI were not (). Thus, we can conclude that only two inflammation-based prognostic scores, NLR and PI, are able to serve as independent risk factors in gastric cancer OS.
Correlation between prognostic scores, clinicopathological features, and DFS rate
Multivariate analysis on DFS time indicated that TNM stage (95% CI, 2.507–4.524; P<0.001), degree of differentiation (95% CI, 0.413–0.838; P=0.003), weight loss (95% CI, 1.064–2.022; P=0.019), GPS (95% CI, 1.015–1.650; P=0.038), NLR (95% CI, 1.119–2.723; P=0.014), PI (95% CI, 1.236–2.182; P=0.001), and adjuvant chemotherapy (95% CI, 0.290–0.582; P<0.001) were independent risk factors for the recurrence of gastric carcinoma after surgery (). These markers are consistent with the correlation between prognostic scores, clinicopathological features, and OS rate, with the exception of weight loss, suggesting that the inflammation-based prognostic scores are consistent and may be used for OS as well as DFS rate.
Comparison of inflammation-based scores and TNM staging system
The LT and LR chi-square test were used to determine homogeneity, discriminatory ability, and monotonicity of gradients for inflammation-based scores (GPS, NLR, PLR, PI, and PNI) and TNM stage to compare the prognostic value of both in patients with gastric cancer. The results suggest that GPS prognosis was comparable to TNM stage. Both had significantly better homogeneity (higher LR χ2), discriminatory ability, and monotonicity of gradients (higher LT χ2) as compared to NLR, PLR, PI, and PNI (). ROC curve analysis indicated that GPS and TNM stage had a markedly larger area under the curve than other inflammation-based prognostic scores (, ). Overall, we demonstrated that GPS and TNM stage had similar prognostic value in patients with gastric cancer, and are superior to NLR, PLR, PI, and PNI scores.
Figure 2 Comparison of the area under the ROC curve for outcome prediction between the inflammation-based prognostic scores at (A) 1 year, (B) 3 years, and (C) 5 years in patients with GPS, TNM, NLR, PLR, PI, and PNI.
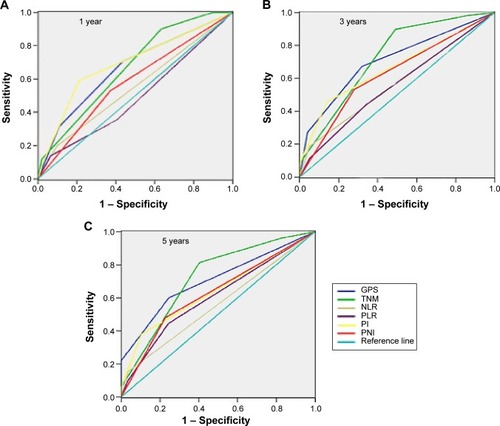
Table 3 The LT and LR chi-square test for inflammation-based prognostic score
Table 4 Comparison of the AUC between inflammation-based prognostic scores and TNM stage
Subgroup survival analysis of GPS scores and TNM stages in patients with or without adjuvant chemotherapy
Since our result showed that the prognostic values of GPS and TNM stage were superior to other inflammation-based scores, we performed subgroup survival analysis to examine whether adjuvant chemotherapy increased or decreased survival in a subgroup of patients with a specific GPS score and TNM stage. No patients in TNM stage I received adjuvant chemotherapy. However, adjuvant chemotherapy significantly increased the OS and DFS rates in patients with TNM stage II, III, and IV gastric cancer (). Conversely, subgroup analysis of GPS scores suggested that adjuvant chemotherapy significantly prolonged the OS and DFS time in patients with GPS score of 0, but not with GPS score of 1 and 2 (). Therefore, adjuvant therapy significantly increased overall and disease-free survival in patients with stage II, III, and IV gastric cancer, as well as in patients with a low inflammation-based prognostic score at onset.
Table 5 Subgroup survival analysis of GPS scores and TNM stages in patients with or without adjuvant chemotherapy
Correlation between clinicopathological features and GPS score
Statistical analysis showed that weight loss (P=0.041), NLR (P<0.001), PNI (P<0.001), PI (P<0.001), and TNM stage (P<0.001) were significantly different among patients with differential GPS scores (Pan QX, unpublished data, 2014). Compared to a GPS score of 0, patients with GPS score of 1 and 2 had more significant weight loss, higher NLR score, higher TNM stage, and less differentiated tumors. There was no statistically significant correlation between GPS scores and age, sex, carcinoembryonic antigen, PLR, histological type of cancer, differentiation, cancer volume, and position (Pan QX, unpublished data, 2014). Therefore, patients with a higher GPS score tended to have more severe disease, which we showed did not respond well to adjuvant chemotherapy.
Discussion
The aim of our retrospective study was to compare the systemic inflammation-based prognostic scores to the TNM stage and thus identify clinically useful prognostic factors for individualized treatment in patients undergoing gastric cancer resection. Our study has determined the GPS value to be comparable to TNM stage, suggesting that both are important markers for prognosis in patients undergoing resection. Both GPS and TNM stage are superior predictors of cancer survival as compared to NLR, PLR, PI, and PNI inflammation-based prognostic scores in patients undergoing resection of gastric cancer. These markers in combination may effectively provide individualized prognostic support for gastric cancer patients.
Previous studies have shown that NLR is an independent prognostic factor for both early and late stages of gastric cancer.Citation29,Citation34,Citation35 Shimada et alCitation30 have demonstrated that a high preoperative NLR score correlates with a poor prognosis in gastric cancer. However, this finding is controversial. Lee et alCitation36 have shown that NLR and PLR are correlated to OS but not to cancer recurrence in patients with advanced gastric cancer receiving chemotherapy. Based on an analysis of 324 patients with stage III gastric cancer, Deshen et al showed that NLR was not an independent prognostic factor following surgery.Citation32 However, the differences in these studies may be due to the subset of preoperative and postoperative patients, as well as the specific stages of gastric cancer in the patients analyzed. In addition to NLR score, the optimal threshold score of neutrophil-based NLR is also controversial in the field of gastric cancer. While the above studies have shown that the threshold of NLR score is 2.5–5, Shimada et alCitation30 performed a retrospective high-quality study in 1,028 patients with gastric cancer and suggested the optimal NLR threshold to be 3–4. Our study also confirmed that NLR thresholds of 3 and 4 were clinically meaningful to predict survival. Using ROC curve analysis, we have shown that an NLR score of 4 is the optimal threshold to predict survival after surgery, suggesting that a higher neutrophil to lymphocyte ratio, consistent with increased inflammation, is more predicative of survival after surgery. Similar to other studies, we have demonstrated that NLR is an independent risk factor in Chinese patients with gastric cancer.
PLR has been shown to be an independent risk factor in other malignant cancers, such as pancreatic ductal adenocarcinoma,Citation21 colon cancer,Citation22 and ovarian cancer.Citation37 Studies from Dutta et alCitation31 and Deshen et alCitation32 have suggested that PLR does not have an independent prognostic value in gastric cancer, which we also confirm in the present study. However, as thrombocytosis has been demonstrated to be an independent risk factor in gastric cancer,Citation38 and is related to platelet ratio, it may be worthwhile to perform larger-scale studies to confirm whether PLR is an independent prognostic factor in gastric cancer.
While multiple studies have suggested a prognostic value for PI and PNI in various cancers such as lung cancer,Citation23 prostate cancer,Citation24 and hepatocellular cancer,Citation25 whether PI and PNI are risk factors in gastric cancer remains unclear. To the best of our knowledge, our current study is the first to show that preoperative PI has significant prognostic value and is an independent risk factor for the recurrence of carcinoma in patients with gastric cancer. Therefore, this marker in combination with other inflammatory markers may provide enhanced value for the prognosis of individual patients with gastric cancer. Although we found that an elevated preoperative PNI is also associated with poor survival in a univariate analysis, we determined it is not an independent prognostic indicator.
The GPS, an inflammation-based prognostic score based on serum CRP and albumin levels, has been implicated as a prognostic factor in various malignant solid tumors, including liver cancer,Citation17,Citation18 non-small-cell lung cancer,Citation11,Citation12 colorectal cancer,Citation15 ovarian cancer,Citation14 and renal cancer.Citation16 However, studies of GPS in the prognosis of gastric cancer are relatively rare because CRP is not routinely examined during treatment. Crumley et al have demonstrated that GPS is an effective and simple predictor of survival in patients with inoperable gastro-esophageal cancer.Citation39 In addition, a high GPS level was significantly correlated with poor prognosis in these patients with inoperable cancer. The authors further demonstrated that GPS was an independent risk factor in patients undergoing potentially curative resection of gastric cancer, while NLR and PLR were not.Citation31 Deshen et alCitation32 suggested a similar conclusion in patients with stage III gastric cancer who have undergone surgery. However, these conclusions did not include PI, PNI, and the most commonly used TNM staging system for comparison of prognostic value in patients with gastric cancer. Our study demonstrates that GPS, in addition to NLR, PI, and TNM stage, was an independent predictor of survival after surgery in patients with gastric cancer, as shown by multivariate analysis. Therefore, we were able to determine the GPS correlation in addition to several new correlations for prognostic values that have not been analyzed in concert previously. Additionally, the present study demonstrated that preoperative serum CRP, albumin, PLR, NLR, PI, PNI, and TNM stage were associated with OS and DFS. Patients with high CRP level, hypoalbuminemia, advanced TNM stage, and high scores of inflammation-based markers had poor OS and DFS both 3 and 5 years after surgery. Similar to the studies done in other solid tumors,Citation24,Citation26–Citation28 the GPS score performed similarly to the TNM stage, and had significant higher prognostic value than NLR, PLR, PI, and PNI in patients with gastric cancer. To the best of our knowledge, our research is the first to include PI and PNI in the comparison of inflammation-based prognostic scores in patients with gastric cancer. Also, our study is the first to quantitatively compare the prognostic value of inflammation-based scores and TNM staging system in patients with gastric cancer.
Whether patients with gastric cancer will benefit from adjuvant chemotherapy after surgery remains controversial. While one study suggested that postoperative adjuvant chemotherapy was only able to increase survival in patients of earlier stages,Citation40 the CLASSIC research indicated that adjuvant chemotherapy increases survival in both earlier and advanced stages.Citation41 Our study showed that adjuvant chemotherapy significantly increased OS and DFS in both early and advanced TNM stages of gastric cancer. However, we found that adjuvant chemotherapy only benefited patients with preoperative GPS scores of 0, but not those with scores of 1 or 2, which is consistent with a similar study conducted in lung cancer.Citation23 Patients with elevated GPS scores have already presented with malnutrition and increased systemic inflammatory response, which is known to increase the toxicity of adjuvant chemotherapy by inhibiting drug metabolism.Citation23,Citation42 Therefore, these patients respond less to the adjuvant chemotherapy treatment and do not benefit from it. Recent studies also suggested that the addition of anti-inflammatory drugs during chemotherapy may be a new effective treatment to increase patient survival.Citation43 However, the mechanisms of how systemic inflammatory response affects adjuvant chemotherapy need to be further clarified in the future.
Besides increased NLR, PN, PI, and TNM stage, we found that elevated GPS was associated with increased weight loss in the patients. This is consistent with previous studies that describe how the systemic inflammatory response is related to progressive functional and nutritional decline as well as increased toxicity of adjuvant chemotherapy in cancer patients that can lead to a subsequent poor outcome.Citation44–Citation47 Our study confirmed that weight loss was associated with poor survival in patients who underwent resection for gastric cancer.
A few limitations should be recognized in this study, including small sample size, a single-center study, and short follow-up time. Larger-scale, multicenter, and prospective studies are therefore needed to confirm the results. In summary, the present study has demonstrated that GPS is an independent predictor of OS and DFS in Chinese patients with gastric cancer. Moreover, preoperative elevated GPS is significantly correlated with poor prognosis. The prognostic value of GPS was comparable to TNM stage and both were superior to other inflammation-based prognostic scores (PLR, NLR, PNI, and PI). We suggest that GPS score and TNM stage can be used together in tandem before surgery to provide more appropriate prediction on survival and more reliable information on treatment decisions for patients with gastric cancer.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Acknowledgments
We thank all the members of the Department of Oncosurgery, First Affiliated Hospital of Fujian Medical University, for their help and support.
Disclosure
The authors report no conflicts of interest in this work.
References
- FerlayJShinHRBrayFGLOBOCAN 2008, cancer incidence and mortality worldwide: IARC Cancer base No 10[EB/OL] Available from: http://globocan.iarc.fr, 2011-02-20
- ZouXNDuanJJHuangpuXMAn analysis of stomach cancer mortality in the national retrospective sampling survey of death causes in China, 2004–2005Chin J Prevent Med Sci2010445390397
- RoxburghCSMcMillanDCRole of system inflammatory response in predicting survival in patients with primary operable cancerFuture Oncol2010614916320021215
- ArgilesJMBusquetsSToledoMLopez-SorianoFJThe role of cytokines in cancer cachexiaCurr Opin Support Palliat Care20093163168
- BalkwillFMantovaniAInflammation and cancer: back to Virchow?Lancet2001357925553954511229684
- CoussensLMWerbZInflammation and cancerNature2002420691786086712490959
- KusumantoYHDamWAHospersGAMeijerCMulderNHPlatelets and granulocytes, in particular the neutrophils, from important compartments for circulating vascular endothelial growth factorAngiogenesis20036428328715166496
- UlichTRDelCJGuoKIn vivo hematologic effects of recombinant interleukin-6 on hematopoiesis and circulating numbers of RBCs and WBCsBlood1989731081102783370
- PhilipMRowleyDASchreiberHInflammation as a tumor promoter in cancer inductionSemin Cancer Biol20041443343915489136
- HeikkilaKEbrahimSLawlorDAA systematic review of the association between circulating concentrations of C reactive protein and cancerJ Epidemiol Commun Health200761824833
- ForrestLMMcMillanDCMcArdleCSComparison of an inflammation-based prognostic score (GPS) with performance status (ECOG) in patients receiving platinum-based chemotherapy for inoperable non-small-cell lung cancerBr J Cancer2004901704170615150622
- ForrestLMMcMillanDCMcArdleCSA prospective longitudinal study of performance status, an inflammation-based prognostic score (GPS) and survival in patients with inoperable non-small-cell lung cancerBr J Cancer2005921834183615870712
- ProctorMJMorrisonDSTalwarDA comparison of inflammation-based prognostic score in patients with cancer. A Glasgow inflammation outcome studyEur J Cancer20112031101106
- SharmaRHookJKumarMGabraHEvaluation of an inflammation-based prognostic score in patients with advanced ovarian cancerEur J Cancer20084425125618155897
- SharmaRZucknickMLondonRKacevskaMLiddleCClarkeSJSystemic inflammatory response predicts prognosis in patients with advanced-stage colorectal cancerClin Colorectal Cancer2008733133718794066
- RamseySLambGWAitchisonMGrahamJMcMillanDCEvaluation of an inflammation-based prognostic score in patients with metastatic renal cancerCancer200710920521217149754
- HuangJXuLLuoYHeFZhangYChenMThe inflammation-based scores to predict prognosis of patients with hepatocellular carcinoma after hepatectomyMed Oncol201431488324535607
- IshizukaMKubotaKKitaJImpact of an inflammation based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patientsAm J Surg2012203110110621429472
- YingHQDengQWHeBSPanYQWangFSunHLThe prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patientsMed Oncol2014311230525355641
- AzabBShahNRadbelJPretreatment neutrophil/lymphocyte ratio is superior to platelet/lymphocyte ratio as a predictor of long-term mortality in breast cancer patientsMed Oncol201330143223283648
- SmithRABosonnetLRaratyMPreoperative platelet–lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinomaAm J Surg2009197446647218639229
- HeWYinCGuoGInitial neutrophil lymphocyte ratio is superior to platelet lymphocyte ratio as an adverse prognostic and predictive factor in metastatic colorectal cancerMed Oncol201330143923307251
- KasymjanovaGMacDonaldNAgulnikJSThe predictive value of pre-treatment inflammatory markers in advanced non-small-cell lung cancerCurr Oncol2010174525820697515
- ProctorMJMorrisonDSTalwarDA comparison of inflammation-based prognostic scores in patients with cancer. A Glasgow inflammation outcome studyEur J Cancer201147172633264121724383
- PinatoDJNorthBVSharmaRA novel, externally validated inflammation-based prognostic algorithm in hepatocellular carcinoma: the prognostic nutritional index (PNI)Br J Cancer201210681439144522433965
- IshizukaMKubotaKKitaJImpact of an inflammation based prognostic system on patients undergoing surgery for hepatocellular carcinoma: a retrospective study of 398 Japanese patientsAm J Surg2012203110110621429472
- KinoshitaAOnodaHImaiNComparison of the prognostic value of inflammation-based prognostic scores in patients with hepatocellular carcinomaBr J Cancer2012107698899322878374
- SumantaDAndrewBGrantMComparison of the prognostic value of tumor- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancerCancer World J Surg20013518611866
- HirashimaMHiguchiSSakamotoKThe ratio of neutrophils to lymphocytes and the phenotypes of neutrophils in patients with early gastric cancerJ Cancer Res Clin Oncol19981243293349692841
- ShimadaHTakiguchiNKainumaOHigh preoperative neutrophil-lymphocyte ratio predicts poor survival in patients with gastric cancerGastric Cancer201013317017620820986
- DuttaSCrumleyABFullartonGMHorganPGMcMillanDCComparison of the prognostic value of tumour and patient related factors in patients undergoing potentially curative resection of gastric cancerAm J Surg2012204329429922444831
- DeshenWChaoRMiaoZQComparison of the prognostic value of various preoperative inflammation-based factors in patients with stage III gastric cancerTumor Biol2012333749756
- MarreroJAFontanaRJBarratAPrognosis of hepatocellular carcinoma: comparison of 7 staging systems in an American cohortHepatology20054170771615795889
- YamanakaTMatsumotoSTeramukaiSIshiwataRNagaiYFukushimaMThe base line ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancerOncology2007733–421522018424885
- JungMRParkYKJeongOElevated preoperative neutrophil to lymphocyte ratio predicts poor survival following resection in late stage gastric cancerJ Surg Oncol2011104550451021618251
- LeeSOhSYKimSHPrognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapyBMC Cancer20131335023876227
- GungorTKanat-PektasMSucakAMollamahmutogluLThe role of thrombocytosis in prognostic evaluation of epithelial ovarian tumorsArch Gynecol Obstet20092791535618470520
- IkedaMFurukawaHImamuraHPoor prognosis associated with thrombocytosis in patients with gastric cancerAnn Surg Oncol20029328729111923136
- CrumleyABMcMillanDCMcKernanMMcDonaldACStuartRCEvaluation of an inflammation-based prognostic score in patients with inoperable gastro-oesophageal cancerBr J Cancer20069463764116479253
- SakuramaltoSSasakoMYamaguchiTAdjuvant chemotherapy for gastric cancer with s-1, an oral fluoropyrimidineN Engl J Med2007357181810182017978289
- BangYJKimYWYangHKAdjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trialLancet201237931532122226517
- ClarkeSJChuaMMooreMUse of inflammatory markers to guide cancer treatmentClin Pharmacol Ther20119047547821775983
- AggarwalBBVijayalekshmiRVSungBTargeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foeClin Cancer Res20091542544019147746
- McMillanDCScorrHRWatsonWSLongitudinal study of body cell mass depletion and the inflammatory response in cancer patientsNutr Cancer1998311011059770720
- AndreyevHNormanAROatesJCunninghamDWhy do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies?Eur J Cancer1998345035099713300
- StephensMRLewisWGWhiteSPrognostic significance of alarm symptoms in patients with gastric cancerBr J Surg200592784084615892157
- CostaMLde Cassia Braga RibeiroKMachadoMACostaACMontagniniALPrognostic score in gastric cancer: the importance of a conjoint analysis of clinical pathologic and therapeutic factorsAnn Surg Oncol200613684385016614885
