Abstract
Radiotherapy is a powerful cure for local advanced non-small cell lung cancer. However, radioresistance and tumor relapse still occur in a high proportion of patients. Octamer-4 (Oct-4), a transcription factor of the POU family, plays a key role in maintaining chemoradioresistant properties and regulating cancer progression. In this study, we demonstrated that Oct-4 expression was significantly increased in radioresistant H460 (H460R) cell line. CpG oligodeoxyribonucleotide (CpG-ODN) 7909 sensitized H460R cells when combined with irradiation treatment. The clonogenic capacity was significantly decreased, and the values of D0 and Dq were lower than those of irradiation alone group. The sensitive enhancement ratio (SER) of D0 was 1.224. This combined treatment led to a dramatic reduction in Oct-4 expression in a dose-dependent manner and also showed increased percentage of cells in the radiosensitive G2/M phase relative to either treatment alone. These results identified that Oct-4 was involved in radioresistance. CpG-ODN 7909 could enhance radiosensitivity partly through downregulating Oct-4 expression in radioresistant lung cancer cells.
Introduction
Lung cancer remains lethal with 5-year survival rate generally lower than 20%, despite intensive therapeutic efforts.Citation1 As a powerful cure for local advanced non-small cell lung cancer (NSCLC), radiotherapy remains largely palliative like most other antitumor therapies due to the adaptive resistance.Citation2
Octamer-4 (Oct-4), a transcription factor of the POU family, plays a key role in the maintenance of embryonic stem cell proliferation and pluripotency.Citation3,Citation4 Moreover, Oct-4 is also overexpressed in some solid tumors, including breast, bladder, colorectal, renal, and lung cancers, which is associated with the maintenance of cancer stem-like and chemoradioresistant properties.Citation5–Citation12 The ectopic expression of Oct-4 controls epithelial–mesenchymal transition and plays an important role in enhancing malignancy and regulating cancer progression.Citation5,Citation8,Citation9,Citation13–Citation15
Toll-like receptor 9 (TLR9) has been identified to be the receptor conferring CpG reactivity by directly engaging bacterial DNA or synthetic CpG oligodeoxyribonucleotides (CpG-ODNs) in a CpG motif-dependent manner.Citation16–Citation20 Owing to the immunostimulatory effects, CpG-ODNs targeting TLR9 have been considered as antitumor drugs, as monotherapy or in combination with radiotherapy, chemotherapy, or novel biological treatment.Citation21 In previous studies, we demonstrated that CpG-ODNs present radiation-enhancing effects when combined with radiotherapy, which predominantly attributes to the release of cytokines, activation of natural killer cells, and induction of T-cell responses.Citation22–Citation24 Because of the overexpression of TLR9 in radiation-resistant human lung adenocarcinoma cell line, CpG-ODNs could even present significant radiosensitizing effects in radioresistant cells in vitro.Citation25 However, whether CpG-ODNs affect the cancer stem-like properties of radioresistant lung cancer cells has yet to be elucidated. Accordingly, in the present study, we focus on the effects of CpG-ODNs on Oct-4 expression in radiation-resistant lung cancer cell line.
Materials and methods
Cell culture
The human NCI-H460 NSCLC cell line was purchased from Chinese Academy of Sciences (Shanghai, People’s Republic of China) and was cultured in RPMI-1640 medium (HyClone, MA,USA) supplemented with 10% fetal bovine serum at 37°C in a humidified atmosphere of 5% CO2.
Reagents
CpG-ODN 7909 (5′-TCGTCGTTTTGTCGTTTTGT CGTT-3′-) was selected according to the published reports and purchased from Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. (Shanghai, People’s Republic of China).Citation23,Citation25,Citation26 Chloroquine (CQ), a TLR9 inhibitor, was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). CpG-ODN 7909 and CQ were separately resuspended in phosphate buffer saline to 1 mg/mL stock solutions and maintained at −20°C until use.
Generation of radioresistant cell line
H460 cells in an exponentially growing phase were irradiated at a dose of 6.37 Gy using a high-energy 6 MV X-ray accelerator delivering 2 Gy/min (source-to-surface distance =100 cm). For the generation of radioresistant variant cell line, cells were treated for ten rounds, and this radioresistant cell line was designated as radioresistant H460 (H460R).Citation25
Study design
H460R cells were divided into six groups and plated in six-well plates (1.5×105/well): control group, CpG-ODN 7909 group (CpG group), irradiated group (IR group), irradiated + CpG-ODN 7909 group (IR + CpG group), irradiated + CpG-ODN 7909 + CQ group (IR + CpG + CQ group), and irradiated + CQ group (IR + CQ group). CpG-ODN 7909 was administrated to a final concentration of 20 µg/mL for 24 hours. CQ was used at a final concentration of 10 µg/mL in pretreatment during 1 hour prior to stimulation with CpG-ODN 7909. Then, cells were irradiated with 10 Gy. Logarithmic growing cells of each group were harvested at 48 hours after irradiation (IR).
Clonogenic survival assay
Cells that received different treatments were irradiated with graded IR doses ranging from 0 Gy to 8 Gy and then immediately trypsinized and seeded in triplicate in six-well plates (500–3,000 cells per well depending on the expected surviving fraction after IRCitation27). After 10 days of incubation, cells were fixed and stained with crystal violet, and colonies ≥50 cells were counted. Clonogenic survival fraction was calculated as (irradiated cell colony numbers/unirradiated cell colony numbers) ×100%. Data were analyzed with linear quadratic and single-hit multitarget models.
Western blot analysis
Western blot analysis was used to determine the expression of Oct-4. The whole cell lysate was prepared using the sodium dodecyl sulfate (SDS) lysis buffer (Beyotime, Nantong, People’s Republic of China) with 1 mM phenylmethanesulfonyl fluoride (Beyotime). Protein concentration was determined using Coomassie blue fast staining solution (Beyotime). Protein extracts were separated by 10% SDS-polyacrylamide gel electrophoresis, transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA), blocked with 5% nonfat dry milk in tris-buffered saline (TBS) containing 0.1% Tween-20 (TBS-T) for 2 hours at room temperature, and then incubated with the following primary antibodies at 4°C overnight: anti-Oct-4 (Abcam, Cambridge, UK) and anti-β-actin (Santa Cruz, Dallas, TX, USA). Membranes were washed with TBS-T three times and probed with secondary antibodies for 1 hour at room temperature. Protein bands were visualized using chemiluminescence HRP substrate (EMD Millipore).
Cell cycle assay
The cell cycle was analyzed by measuring the DNA content stained with propidium iodide in the presence of RNase (BD Biosciences, San Jose, CA, USA) per manufacturer’s instructions. The cells were harvested and fixed with 75% ethanol at 4°C overnight and then incubated with 500 µL propidium iodide/RNase staining buffer (BD Biosciences) at room temperature for 15 minutes. The percentage of cells at each cell cycle was evaluated by a flow cytometer.
Statistical analysis
SPSS 21.0 and GraphPad Prism 5.0 were used for data analysis, and the results were presented as mean ± standard deviation. A P-value <0.05 was considered statistically significant difference.
Results
Generation of radioresistant lung cancer cells
The responses to IR between resistant cells and parental cells were analyzed by clonogenic assay. As shown in and , in the resistant H460R cells, a marked increase in D0 and N values and a broader initial shoulder were observed as compared to parental H460 cells. H460R cells were more resistant to cell death post-IR at each dose tested. These results indicated the acquired radioresistant characteristics of H460R cells.
Figure 1 Analysis of radiosensitivity via clonogenic assay.
Abbreviations: H460R, radioresistant H460; IR, irradiation.
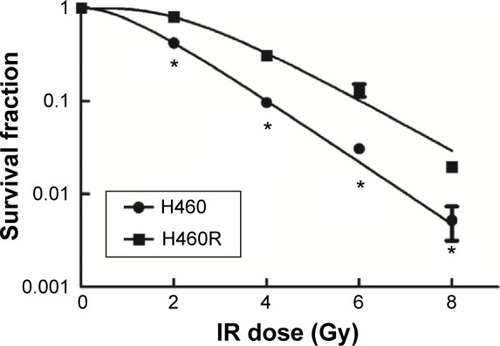
Table 1 Comparison of radiosensitivity between H460 cells and H460R cells
Elevated expression of Oct-4 in radioresistant lung cancer cells
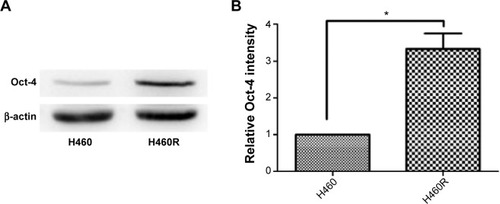
Since Oct-4 expression plays an important role in regulating cancer progression and maintaining chemoradioresistant properties, we detected the level of Oct-4 in H460 and H460R cells. As shown in , a substantial increase in Oct-4 expression was detected in H460R cells as compared to parental H460 cells, suggesting that Oct-4 was involved in radioresistance, and therefore, the radioresistant lung cancer cells may present more aggressive phenotypes than parental cells.
Figure 2 The expression level of Oct-4 was higher in H460R cells compared with H460 cells.
Abbreviations: Oct-4, octamer-4; H460R, radioresistant H460.
Effects of CpG-ODN 7909 on expression of Oct-4 post-IR in radioresistant lung cancer cells
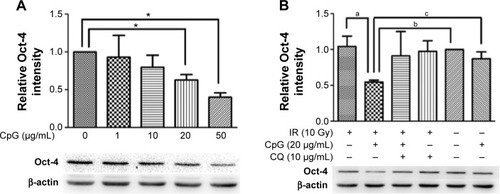
To determine whether CpG-ODN 7909 influences the expression of Oct-4, we treated H460R cells with an increasing concentration of CpG-ODN 7909 for 24 hours, exposed them to 10 Gy IR, and then incubated them for 48 hours. We then detected that, when combined with IR, CpG-ODN 7909 downregulated Oct-4 expression in H460R cells in a dose-dependent manner. Pretreatment of H460R cells with CQ caused a blockade in this reduction (). These results indicated that CpG-ODN 7909 could reduce the Oct-4 expression through TLR9 signaling and may further influence the radiosensitivity of radioresistant lung cancer cells.
Figure 3 CpG-ODN 7909 downregulated Oct-4 expression in H460R cells when combined with IR.
Abbreviations: CpG-ODN 7909, CpG oligodeoxyribonucleotide 7909; Oct-4, octamer-4; H460R, radioresistant H460; IR, irradiation; CQ, chloroquine; IR group, irradiated group.
Effects of CpG-ODN 7909 on the radiosensitivity of radioresistant lung cancer cells
To assess and characterize the radiation-enhancing effects of CpG-ODN 7909, clonogenic survival analysis was performed. As shown in and , CpG-ODN 7909 presented significant radiation-enhancing effects. H460R cells treated with CpG-ODN 7909 plus IR exhibited decreased clonogenic capacity and displayed lower values of D0, Dq, and N than those of IR alone group, and the sensitive enhancement ratio (SER) of D0 was1.224. However, after the activation of TLR9 was blocked by CQ, we were unable to detect any radiation-enhancing effects.
Figure 4 CpG-ODN 7909 presented significant radiation-enhancing effects on H460R cells.
Abbreviations: CpG-ODN 7909, CpG oligodeoxyribonucleotide 7909; H460R, radioresistant H460; IR, irradiation; TLR9, toll-like receptor 9; CQ, chloroquine.
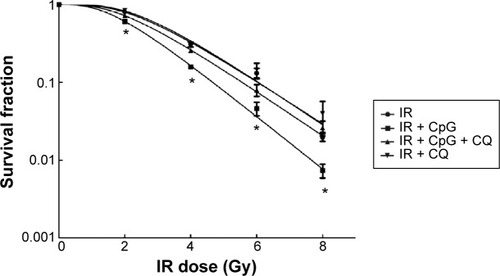
Table 2 Comparison of radiosensitivity of H460R cells treated with IR, CpG-ODN 7909, or CQ
Effects of CpG-ODN 7909 on cell cycle regulation after IR in radioresistant lung cancer cells
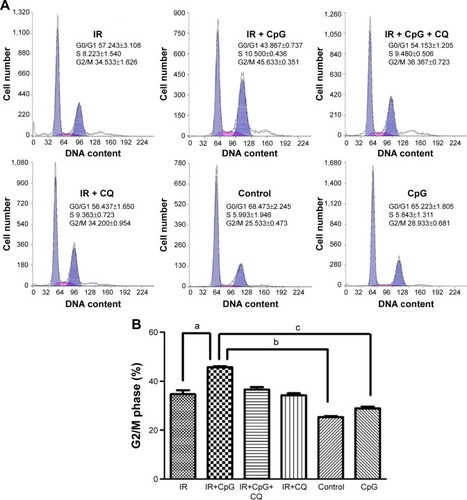
To determine if radiosensitizing effects of CpG-ODN 7909 were related to cell cycle regulation, we performed flow cytometric analysis to determine the number of cells in each cell cycle phase. As shown in , combined treatment with IR and CpG-ODN 7909 showed an increased percentage of cells in G2/M phase compared to either treatment alone in H460R cells, which could be blocked by the co-treatment of CQ. The results above suggested that CpG-ODN 7909 allowed H460R cells to produce G2/M arrest through TLR9 signaling.
Figure 5 Effect of CpG-ODN 7909 on G2/M phase arrest in H460R cells.
Abbreviations: CpG-ODN 7909, CpG oligodeoxyribonucleotide 7909; H460R, radioresistant H460; IR, irradiation; CQ, chloroquine; TLR9, toll-like receptor 9.
Discussion
Lung cancer is the most common diagnosed tumor with a poor prognosis.Citation1 Although radiotherapy has been a powerful cure for locally advanced NSCLC, its efficiency remains limited as tumors develop adaptive response to radiotherapy and become more aggressive, resistant, and invasive.Citation2 As a positive regulator of tumor dedifferentiation, Oct-4 plays an important role in maintaining the cancer stem-like and chemoradioresistant properties.Citation10,Citation12 In the present study, we found that the resistant H460R cells showed a significant increase in Oct-4 expression relative to parental cells, which indicated that Oct-4 was associated with the radioresistant phenotype. Thus, targeting Oct-4 might be an effective approach to interfere with the properties of cancer stem-like cells (CSCs) and improve the efficacy of cancer treatments.
Due to the immune stimulatory effects, CpG-ODNs have been widely used in cancer therapy, eg, treating oral squamous cell carcinoma, brain tumor, breast cancer, and lung cancer.Citation28–Citation31 In our previous studies, we have demonstrated that CpG-ODNs could enhance apoptosis, induce prolonged G2/M arrest, and stimulate TNF-α secretion in human NSCLC cells and thereby enhanced radiosensitivity. As TLR9 was overexpressed in radiation-resistant NSCLC cells, CpG-ODNs could even present significant radiosensitizing effects in radioresistant cells in vitro.Citation23,Citation25 All these results urged us to further explore the sensitization mechanism of CpG-ODNs and the influence of CpG-ODNs on Oct-4, as Oct-4 is associated with the radioresistant phenotype.
The ectopic expression of Oct-4 controls epithelial–mesenchymal transition and plays an important role in regulating cancer progression and maintaining the chemoradioresistant properties.Citation5,Citation8,Citation9,Citation13–Citation15 In this study, overexpression of Oct-4 was observed in H460R cells. However, CpG-ODNs induced a dramatic reduction in Oct-4 expression in a dose-dependent manner when combined with IR.
TLR9-CpG-DNA interaction is pH dependent and occurs at the acidic pH (6.5–4.5) found in endosomes and lysosomes. CQ, a known TLR9 antagonist, is a weak base that can partition into endosomes and perturb endosomal pH.Citation32,Citation33 CQ has been widely used as the inhibitor for endosomal acidification to inhibit the activation of TLR9.Citation34–Citation37 In the present study, pretreatment of H460R cells with CQ caused a blockade in the reduction of Oct-4 expression induced by CpG-ODNs, suggesting that CpG-ODNs could reduce the Oct-4 expression through TLR9 signaling and may therefore present radiosensitizing effects. Cell cycle phase determines cells’ relative radiosensitivity, with cells being most sensitive in the G2/M phase.Citation38 As shown above, combined treatment with IR and CpG-ODNs led to the accumulation of cells in the G2/M phase. Once the activation of TLR9 was blocked by CQ, the CpG-ODN-induced G2/M arrest was abrogated. These results further demonstrated that CpG-ODNs sensitized H460R cells when combined with radiotherapy through TLR9 signaling. As shown in , the SER of D0 was about 1.224.
In summary, Oct-4 was involved in radioresistance. Combined treatment with CpG-ODNs and IR presented significant radiosensitizing effects partly through downregulating Oct-4 expression. As cancer stem-like cells phenotype is a dynamic process and can be acquired through Oct-4-mediated dedifferentiation,Citation7,Citation11,Citation12 further investigation remains to be performed to study the role of Oct-4 and the related signaling pathway in radioresistance and to explore the direct effect of CpG-ODNs on CSCs.
Acknowledgments
This work was supported by the Scientific Fund of the Shanghai Municipal Health Bureau.
Disclosure
The authors report no conflicts of interest in this work.
References
- AllemaniCWeirHKCarreiraHCONCORD Working GroupGlobal surveillance of cancer survival 1995–2009: analysis of individual data for 25 676 887 patients from 279 population-based registries in 67 countries (CONCORD-2)Lancet20143859972977101025467588
- KimJJTannockIFRepopulation of cancer cells during therapy: an important cause of treatment failureNat Rev Cancer20055751652515965493
- NiwaHMiyazakiJSmithAGQuantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cellsNat Genet200024437237610742100
- BabaieYHerwigRGreberBAnalysis of Oct4-dependent transcriptional networks regulating self-renewal and pluripotency in human embryonic stem cellsStem Cells200725250051017068183
- LiuCGLuYWangBBClinical implications of stem cell gene Oct-4 expression in breast cancerAnn Surg201125361165117121394007
- AtlasiYMowlaSJZiaeeSABahramiAROCT-4, an embryonic stem cell marker, is highly expressed in bladder cancerInt J Cancer200712071598160217205510
- WenKFuZWuXFengJChenWQianJOct-4 is required for an antiapoptotic behavior of chemoresistant colorectal cancer cells enriched for cancer stem cells: effects associated with STAT3/SurvivinCancer Lett20133331566523340171
- KimKRoJYKimSChoYMExpression of stem-cell markers OCT-4 and CD133: important prognostic factors in papillary renal cell carcinomaHum Pathol201243122109211622944295
- ChenZWangTCaiLClinicopathological significance of non-small cell lung cancer with high prevalence of Oct-4 tumor cellsJ Exp Clin Cancer Res2012311022300949
- ChenYCHsuHSChenYWOct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cellsPLoS One200837e263718612434
- HuTLiuSBreiterDRWangFTangYSunSOctamer 4 small interfering RNA results in cancer stem cell-like cell apoptosisCancer Res200868166533654018701476
- KumarSMLiuSLuHAcquired cancer stem cell phenotypes through Oct4-mediated dedifferentiationOncogene201231474898491122286766
- WangDLuPZhangHOct-4 and Nanog promote the epithelial-mesenchymal transition of breast cancer stem cells and are associated with poor prognosis in breast cancer patientsOncotarget2014521108031081525301732
- ChiouSHWangMLChouYTCoexpression of Oct4 and Nanog enhances malignancy in lung adenocarcinoma by inducing cancer stem cell-like properties and epithelial-mesenchymal transdifferentiationCancer Res20107024104331044421159654
- HuangPChenJWangLImplications of transcriptional factor, OCT-4, in human bladder malignancy and tumor recurrenceMed Oncol201229282983421533858
- VollmerJKriegAMImmunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonistsAdv Drug Deliv Rev200961319520419211030
- HemmiHTakeuchiOKawaiTA toll-like receptor recognizes bacterial DNANature2000408681374074511130078
- BauerSKirschningCJHäckerHHuman TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognitionProc Natl Acad Sci U S A200198169237924211470918
- CornélieSHoebekeJSchachtAMDirect evidence that toll-like receptor 9 (TLR9) functionally binds plasmid DNA by specific cytosine-phosphate-guanine motif recognitionJ Biol Chem200427915151241512914736866
- LatzESchoenemeyerAVisintinATLR9 signals after translocating from the ER to CpG DNA in the lysosomeNat Immunol20045219019814716310
- HoltickUScheulenMEvon Bergwelt-BaildonMSWeihrauchMRToll-like receptor 9 agonists as cancer therapeuticsExpert Opin Investig Drugs2011203361372
- LiRSongYChenWEnhancing radiosensitivity of human pulmonary adenocarcinoma cell line A549 by CpG ODN1826Cancer Biother Radiopharm2011261697621355778
- ZhaLQiaoTYuanSLeiLEnhancement of radiosensitivity by CpG-oligodeoxyribonucleotide-7909 in human non-small cell lung cancer A549 cellsCancer Biother Radiopharm201025216517020423229
- YuanSQiaoTChenWCpG oligodeoxynucleotide 1826 enhances the Lewis lung cancer response to radiotherapy in murine tumorCancer Biother Radiopharm201126220320821539452
- YanLXuGQiaoTChenWYuanSLiXCpG-ODN 7909 increases radiation sensitivity of radiation-resistant human lung adenocarcinoma cell line by overexpression of toll-like receptor 9Cancer Biother Radiopharm201328755956423705865
- WitzigTEWisemanGAMaurerMJA phase I trial of immunostimulatory CpG 7909 oligodeoxynucleotide and 90 yttrium ibritumomab tiuxetan radioimmunotherapy for relapsed B-cell non-Hodgkin lymphomaAm J Hematol201388758959323619698
- ShortSCGiampieriSWorkuMRad51 inhibition is an effective means of targeting DNA repair in glioma models and CD133+ tumor-derived cellsNeuro Oncol201113548749921363882
- RuanMThornKLiuSThe secretion of IL-6 by CpG-ODN-treated cancer cells promotes T-cell immune responses partly through the TLR-9/AP-1 pathway in oral squamous cell carcinomaInt J Oncol20144462103211024676671
- JarryUDonnouSVincentMTreg depletion followed by intracerebral CpG-ODN injection induce brain tumor rejectionJ Neuroimmunol20142671–2354224369298
- XiaYGuptaGKCastanoAPMrozPAvciPHamblinMRCpG oligodeoxynucleotide as immune adjuvant enhances photodynamic therapy response in murine metastatic breast cancerJ Biophotonics2014711–1289790523922221
- SorrentinoRMorelloSForteGB cells contribute to the antitumor activity of CpG-oligodeoxynucleotide in a mouse model of metastatic lung carcinomaAm J Respir Crit Care Med2011183101369137921278302
- MellmanIFuchsRHeleniusAAcidification of the endocytic and exocytic pathwaysAnnu Rev Biochem1986556637002874766
- RutzMMetzgerJGellertTToll-like receptor 9 binds single-stranded CpG-DNA in a sequence- and pH-dependent mannerEur J Immunol20043492541255015307186
- FiolaSGosselinDTakadaKGosselinJTLR9 contributes to the recognition of EBV by primary monocytes and plasmacytoid dendritic cellsJ Immunol201018563620363120713890
- KuznikABencinaMSvajgerUJerasMRozmanBJeralaRMechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolinesJ Immunol201118684794480421398612
- TillackKBreidenPMartinRSospedraMT lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responsesJ Immunol201218873150315922351936
- SanjuanMARaoNLaiKTCpG-induced tyrosine phosphorylation occurs via a TLR9-independent mechanism and is required for cytokine secretionJ Cell Biol200617271057106816567503
- PawlikTMKeyomarsiKRole of cell cycle in mediating sensitivity to radiotherapyInt J Radiat Oncol Biol Phys200459492894215234026
