Abstract
Aim
To retrospectively evaluate the safety and efficacy of ultrasound-guided percutaneous microwave ablation (MWA) combined with simultaneous transarterial chemoembolization (TACE) in the treatment of patients with advanced intrahepatic cholangiocarcinoma (ICC).
Methods
All patients treated with ultrasound-guided percutaneous MWA combined with simultaneous TACE for advanced ICC at our institution were included. Posttreatment contrast-enhanced computed tomography and/or magnetic resonance imaging were retrieved and reviewed for tumor response to the treatment. Routine laboratory studies, including hematology and liver function tests were collected and analyzed. Procedure-related complications were reviewed and survival rates were analyzed.
Results
From January 2011 to December 2014, a total of 26 advanced ICC patients were treated at our single institute with ultrasound-guided percutaneous MWA combined with simultaneous TACE. There were 15 males and eleven females with an average age of 57.9±10.4 years (range, 43–75 years). Of 26 patients, 20 (76.9%) patients were newly diagnosed advanced ICC without any treatment, and six (23.1%) were recurrent and treated with surgical resection of the original tumor. The complete ablation rate was 92.3% (36/39 lesions) for advanced ICC. There were no major complications observed. There was no death directly from the treatment. Median progression-free survival and overall survival were 6.2 and 19.5 months, respectively. The 6-, 12-, and 24-month survival rates were 88.5%, 69.2%, and 61.5%, respectively.
Conclusion
The study suggests that ultrasound-guided percutaneous MWA combined with simultaneous TACE therapy can be performed safely in all patients with advanced ICC. The complete ablation rate was high and there was no major complication. The overall 24-month survival was 61.5%.
Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second most common primary liver cancer, accounting for 5%–10% of liver malignancies.Citation1 Surgical resection is the current curative treatment; however, at least 75% of the patients were at the advanced stage of disease at the time of diagnosis and lost surgical opportunities.Citation2,Citation3 The median survival for unresectable ICC patients was reported to be about 5–8 months.Citation2,Citation3
Image-guided thermal ablation, including microwave ablation (MWA) and radiofrequency ablation (RFA), has been proved to be an effective and less invasive alternative for the treatment of nonsurgical candidates with liver malignancies owing to its minimal invasiveness, lesser technical demand, lower cost, and repeatability.Citation4 Studies also revealed that thermal ablation could provide long-term survival rates, comparable to those of resection in eligible early-stage hepatocellular carcinoma (HCC) patients.Citation5–Citation7 However, the efficacy of thermal ablation treatment was reported to be limited in those tumors larger than 3 cm or difficult to access.Citation8 The efficacy of thermal ablation is based on heat delivery, and blood flow around the tumor may prevent proper heating. MWA has been reported to be superior to the RFA technique due to its higher ablation rates, wider ablation diameters, lower heat sink effect, and shorter procedure time.Citation9 Transarterial chemoembolization (TACE) is a standard therapy for advanced HCC. Evidence shows that, combined with thermal ablation, the dosages of chemotherapeutic agents and Lipiodol can be significantly reduced for the TACE procedure.Citation10 Studies have also shown that TACE-assisted RFA in patients with medium-sized HCCs may provide better local control and overall survival rates, without significantly increasing the complication rate, comparing to RFA alone.Citation11,Citation12
This study aimed to investigate the safety and efficacy of percutaneous MWA combined with simultaneous TACE in the treatment of advanced ICC.
Patients and methods
Patient selection
This retrospective study was approved by the ethics committee of Zhongshan Hospital. Advanced ICC was defined as stage II and beyond, not suitable for surgical resection based on the American Joint Committee on Cancer’s classification of cholangiocarcinoma.Citation13 Diagnosis of advanced ICC was primarily based on clinical presentation, imaging findings, tumor markers, and pathological examination.Citation14 The inclusion criteria for percutaneous MWA combined with simultaneous TACE treatment were as follows: five or fewer tumors; Eastern Cooperative Oncology Group (ECOG) performance status of patients lower than 2;Citation15 adequate renal function (creatinine level less than 2.0 mg/dL); platelet count greater than 30×109/L; white blood cell count greater than 3×109/L; and prothrombin activity >50%; liver function tests less than 1.5 times the normal upper limit (serum aspartate transaminase <60 U/L, serum alanine transaminase <60 U/L, and total bilirubin concentrations <25.6 mmol/L). Patients with extrahepatic metastases or previous systemic chemotherapy or radiation therapy for ICC were not included in this study.
Ultrasound-guided percutaneous MWA
Percutaneous MWA was performed by using an ECO-100C microwave generator (ECO Medical Equipment Co Ltd, Jiangsu, People’s Republic of China), with maximum power of 100 W. Local anesthesia (1% lidocaine) or general anesthesia was administrated during the procedure. All the lesions were accessed by using ultrasound guidance. According to the manufacturer’s recommendation, single ablation was performed for tumors less than 3 cm in diameter, while multiple overlapping ablations were elected for the lesions ≥3 cm. The electrode was inserted into the tumor to reach the farthest margin of the lesion in order to achieve a negative margin of at least 1 cm. Energy delivery was applied for 8–10 minutes at 60–100 W, and the extent of the ablation area was monitored in real time by ultrasound. After the first ablation, the electrode was withdrawn approximately 2 cm to a second site. The above process was repeated until the entire tumor was adequately covered. At the end of the procedure, the generator was reactivated to heat the needle tract to avoid tumor seeding. For multiple tumors, each lesion suitable for MWA was treated separately with the above technique.
TACE protocol
Routine hepatic and superior mesenteric arteriography were obtained to evaluate vascular anatomy, tumor vascularity, and portal venous patency. After an Rosch hepatic (RH) catheter or microcatheter was inserted into the target vessel, the mixture of chemoagents was injected for chemoembolization (Ultravist 300 3–10 mL, Bayer AG, Leverkusen, Germany; Oxaliplatin 50–100 mg, Jiangsu Hengrui Medicine Co Ltd, Lianyungang, People’s Republic of China; Gemcitabine 600–1,000 mg, Jiangsu Haosen Medicine Co Ltd, Lianyungang, People’s Republic of China; Lipiodol 5–10 mL, Laboratoire Guerbet, Aulnay-sous-Bois, France). The dose of iodized oil mainly depended on the blood supply, tumor size and number, and liver function. Additional gelatin sponge particles (350–510 μm; Ailikang Medicine Co Ltd, Hangzhou, People’s Republic of China) were injected if the target tumor artery did not show stagnant flow after injection of all the emulsion.
Postoperative care
All patients were admitted for supportive care, including antacid agents, antibiotic prophylaxis, liver protection, and antiemetic treatment after the procedure.
Non-contrast computed tomography (CT) scan at days 3–5 after the procedure was used to exclude bleeding, pneumothorax, hydrothorax, ascites, and enterobrosis and preliminarily observe the situation of ablation lesions. Complications were classified according to the practical guidelines of the Society of Interventional Radiology as minor and major complications.Citation16
Imaging follow-up
Following treatment, tumor response was evaluated by using CT and/or magnetic resonance at 1, 3, 6, 9, and 12 months for the first year and every 3–6 months thereafter. Residual viable tumor tissue was considered to be present if enhancement existed at the previously treated tumor at 1-month follow-up, otherwise the patient was regarded as complete ablation. Local tumor recurrence was defined as the presence of peripheral or internal enhanced areas on follow-up imaging more than 1 month after treatment, and/or an increased size of the tumor. Progression of tumor was defined as tumor size increasing at least 20% in the sum of the diameters according to modified RECIST assessment.Citation17 Intrahepatic metastasis was defined as the presence of a new detected lesion. Routine laboratory tests, including complete blood counts, chemistry, tumor markers, and liver function tests, were obtained at each follow-up visit.
Statistical analysis
Progression-free survival was calculated from the date of initial treatment to the time of tumor progression, death or the last follow-up visit. Overall survival was calculated from the start of treatment to the time of death or the last follow-up. The Statistical Package for the Social Sciences (SPSS) program (version 20.0; IBM Corporation, Armonk, NY, USA) was used for statistical analyses. Continuous ordinal data are expressed as means ± standard deviation and qualitative data are expressed as frequency and rate. The Kaplan–Meier method was used to calculate the cumulative survival rates.
Results
Patient characteristics
Between January 2011 and December 2014, a total of 26 consecutive patients were treated with combined percutaneous MWA and simultaneous TACE for advanced ICC. There were 15 males and eleven females, with a mean age of 57.9±10.4 years (range, 43–75 years). lists the baseline characteristics of the 26 patients prior to treatment. Of 26 ICC patients, 20 (76.9%) cases had no prior anticancer treatment, and six (23.1%) were recurrent after surgical resection. Twenty-one patients were treated for a single tumor, and five patients were treated for more than one tumor, with 39 tumors in total. The mean tumor size was 3.6±1.1 cm (range, 2.5–6.5 cm) in diameter. According to Child-Pugh classification, ten (38.5%) cases were class A, and 16 (61.5%) patients were class B.
Table 1 Patient and tumor characteristics
Outcomes
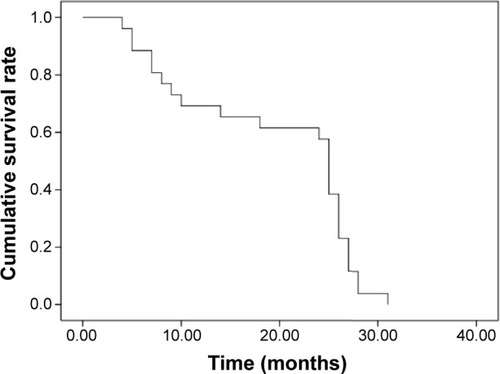
Complete ablations were achieved in 36 lesions in 24 patients (). Residual tumor was identified in two patients on three treated tumors at 1-month follow-up CT. The average number of electrode insertions was two (range, 1–6). Mean follow-up time was 19.2±6.3 months (range, 6–30 months). Deaths occurred due to tumor progression in ten patients and due to other diseases in four patients. Local tumor recurrence occurred in eight patients and intrahepatic/extrahepatic metastases in seven patients. Median progression-free survival was 6.2 months (range, 3–12 months). Median overall survival was 19.5 months (). The 6-, 12-, and 24-month overall survival rates were 88.5%, 69.2%, and 61.5%, respectively.
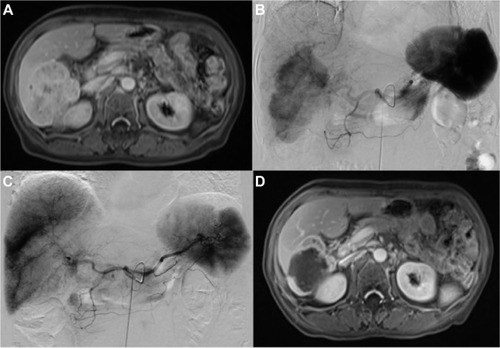
Figure 1 A 63-year-old woman with advanced ICC treated with MWA combined with simultaneous TACE.
Abbreviations: DSA, digital subtraction angiography; MWA, microwave ablation; TACE, transarterial chemoembolization.
Complications
No major complication or procedure-related mortality was observed during the TACE–MWA procedure. The most common minor complications were fever (n=23, 88.5%) and mild-to-moderate pain (n=22, 84.6%). Other rare mild complications included thrombocytopenia (three cases, 11.5%), asymptomatic pleural effusion (two cases, 7.7%), and vomiting (one case, 3.8%). All of these side effects subsided with supportive treatment.
Discussion
ICC is a devastating malignancy with a 5-year survival rate less than 5%, which has not changed significantly over the past 30 years.Citation1 Surgical resection is the current best treatment choice for long-term survival; however, patients usually present with advanced stage of disease at the time of diagnosis, and the treatment options are limited.
Image-guided thermal ablation (MWA or RFA) has been used for the treatment of advanced ICC with encouraging results.Citation18–Citation20 The potential benefits include a relatively high percentage of complete tumor necrosis, minimal invasiveness, easy performance, and repeatability. The therapeutic effect of thermal ablation, however, decreases as the size of tumor increases: the larger the tumor size, the less likely it is to achieve complete tumor necrosis.Citation21 Furthermore, blood supply is usually rich to the tumor, which may reduce the efficacy of thermal ablation due to the undesirable cooling effect.Citation22
Thermal ablation combined with simultaneous TACE has several potential benefits. TACE procedure can effectively decrease heat dispersion during thermal ablation by occluding bloodstream and ultimately promote tumor destruction.Citation23 Meanwhile, thermal ablation may decrease the chemotherapy dose of TACE and consequently reduce adverse reaction, and may also increase the volume of tumor necrosis and prolong progression-free survival. In our study, the average doses of iodized oil and oxaliplatin were 5–10 mL and 50–100 mg, compared to 5–20 mL and 2 mg/kg in the literature for TACE alone. TACE-related complications, such as vomiting and thrombocytopenia, were far below TACE only (3.8% and 11.5%, respectively).Citation24 Besides, TACE is able to detect and deal with some small ICC nodules invisible on CT or magnetic resonance imaging, and some MWA-related comorbidities such as hemorrhage and arteriovenous shunt can be identified and dealt with by TACE in time.Citation25
Previous studies demonstrated that thermal ablation combined with TACE therapy for HCC resulted in a high complete response rate and promising clinical results.Citation12,Citation26,Citation27 Peng et alCitation12 reported that the 1-, 2-, 3-, and 5-year overall survival rates for the groups with RFA and RFA combined with TACE were 89%, 76%, 64%, and 42% and 93%, 83%, 75%, and 50%, respectively. Compared to typical HCC, ICC is commonly hypovascular with a fibrotic nature, which determines the limited efficacy of single TACE therapy. The efficacy of MWA for ICCs smaller than 5.0 cm in diameter has been proved in clinical research; however, MWA combined with simultaneous TACE therapy for advanced ICC has not been described.
In our study, the 6-, 12-, and 24-month overall survival rates were 88.5%, 69.2%, and 61.5%, respectively. The median overall survival was 19.5 months. However, a recent meta-analysis of 14 trials of ICC patients showed a median overall survival of 15.6±1.1 months for TACE treatment only.Citation28 Our results are also significantly better than in those similar patients who were treated at our institution before with TACE only.Citation29 In a retrospective study, Yu et al treated 15 patients with MWA alone.Citation19 The cumulative overall 6-, 12-, and 24-month survival rates were 78.8%, 60.0%, and 60.0%, respectively. Their results are slightly inferior to our combined treatment, which may be due to the small sample size in both studies and different patient selection.
This study revealed no major complication or procedure-related mortality, which may be partially attributed to the reduced dose of chemoagents. All of the minor complications were typically transient and resolved with supportive management. The results of our study suggest that MWA combined with simultaneous TACE therapy was a safe and well-tolerated treatment method for patients with advanced ICC and may have some advantages compared with MWA only, but the conclusion needs further evidence.
The limitations in this study include its retrospective nonrandomized design, relatively insufficient number of subjects, and relatively short follow-up period. Inevitably, these limitations may to some extent influence the reliability of conclusion. Further studies that encompass prospective, randomized controlled design, a large sample size, and long follow-up period are needed to fully evaluate the efficacy of the combination treatment for patients with advanced ICC.
Conclusion
This study suggests that ultrasound-guided percutaneous MWA combined with simultaneous TACE therapy may be a safe option with potential benefits for patients with advanced ICC.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShaibYEl-SeragHBThe epidemiology of cholangiocarcinomaSemin Liver Dis20042411512515192785
- TamandlDHerbergerBGruenbergerBPuhallaHKlingerMGruenbergerTInfluence of hepatic resection margin on recurrence and survival in intrahepatic cholangiocarcinomaAnn Surg Oncol2008152787279418685896
- GusaniNJBalaaFKSteelJLTreatment of unresectable cholan-giocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): a single-institution experienceJ Gastrointest Surg20081212913717851723
- AhmedMSolbiatiLBraceCLInternational Working Group on Image-guided Tumor AblationInterventional Oncology Sans Frontières Expert PanelTechnology Assessment Committee of the Society of Interventional RadiologyStandard of Practice Committee of the CardiovascularInterventional Radiological Society of EuropeImage-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year updateRadiology201427324126024927329
- LencioniRCioniDCrocettiLEarly-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablationRadiology200523496196715665226
- ChenMSLiJQZhengYA prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinomaAnn Surg200624332132816495695
- RautCPIzzoFMarraPSignificant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosisAnn Surg Oncol20051261662815965731
- GarreanSHeringJSaiedAHeltonWSEspatNJRadiofrequency ablation of primary and metastatic liver tumors: a critical review of the literatureAm J Surg200819550852018361927
- BoutrosCSomasundarPGarreanSSaiedAEspatNJMicrowave coagulation therapy for hepatic tumors: review of the literature and critical analysisSurg Oncol201019e22e3219268571
- YamakadoKNakatsukaAOhmoriSRadiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphologyJ Vasc Interv Radiol2002131225123212471186
- MorimotoMNumataKKondoMRadiofrequency ablation combined with transarterial chemoembolization for subcapsular hepatocellular carcinoma: a prospective cohort studyEur J Radiol20138249750323068563
- PengZWChenMSLiangHHA case-control study comparing percutaneous radiofrequency ablation alone or combined with tran-scatheter arterial chemoembolization for hepatocellular carcinomaEur J Surg Oncol20103625726319643561
- EdgeSBByrdDRComptonCCFritzAGGreeneFLTrottiAIIIAJCC Cancer Staging Manual7th edNew YorkSpringer2010
- BridgewaterJGallePRKhanSAGuidelines for the diagnosis and management of intrahepatic cholangiocarcinomaJ Hepatol2014601268128924681130
- SørensenJBKleeMPalshofTHansenHHPerformance status assessment in cancer patients. An inter-observer variability studyBr J Cancer1993677737758471434
- SacksDMcClennyTECardellaJFLewisCASociety of Interventional Radiology clinical practice guidelinesJ Vasc Interv Radiol200314S199S20214514818
- LencioniRLlovetJMModified RECIST (mRECIST) assessment for hepatocellular carcinomaSemin Liver Dis201030526020175033
- YuMALiangPYuXLSonography-guided percutaneous microwave ablation of intrahepatic primary cholangiocarcinomaEur J Radiol20118054855221300500
- LivraghiTMeloniFSolbiatiLZanusGCollaborative Italian Group using AMICA systemComplications of microwave ablation for liver tumors: results of a multicenter studyCardiovasc Intervent Radiol20123586887421833809
- ButrosSRShenoy-BhangleAMuellerPRArellanoRSRadiof-requency ablation of intrahepatic cholangiocarcinoma: feasability, local tumor control, and long-term outcomeClin Imaging20143849049424637151
- LivraghiTLazzaroniSMeloniFRadiofrequency thermal ablation of hepatocellular carcinomaEur J Ultrasound20011315916611369527
- GoldbergSNHahnPFTanabeKKPercutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis?J Vasc Interv Radiol199891011119468403
- SekiTTamaiTNakagawaTCombination therapy with tran-scatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinomaCancer2000891245125111002219
- HerberSOttoGSchneiderJTransarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinomaCardiovasc Intervent Radiol2007301156116517508242
- LiangPWangYYuXDongBMalignant liver tumors: treatment with percutaneous microwave ablation – complications among cohort of 1136 patientsRadiology200925193394019304921
- KimJWKimJHWonHJHepatocellular carcinomas 2–3 cm in diameter: transarterial chemoembolization plus radiofrequency ablation vs radiofrequency ablation aloneEur J Radiol201281e189e19321353417
- ZhaoMWangJPPanCCCT-guided radiofrequency ablation after with transarterial chemoembolization in treating unresectable hepatocellular carcinoma with long overall survival improvementEur J Radiol2012812717272522245655
- EdwardsARayCEJrAbstract No. 247: Transarterial therapies for unresectable cholangiocarcinoma: a meta-analysisJ Vasc Interv Radiol201223S101
- ZhaoQQianSZhuLTranscatheter arterial chemoembolization with gemcitabine and oxaliplatin for the treatment of advanced biliary tract cancerOnco Targets Ther2015859560025792843