Abstract
The third-generation aromatase inhibitors (AIs: anastrozole, letrozole, and exemestane) have now become standard adjuvant endocrine treatment for postmenopausal estrogen receptor-positive breast cancer complementing chemotherapy and surgery. Because of the absence of direct head-to-head comparisons of these AIs, an indirect comparison is needed for individual treatment choice. In this network systemic assessment, the cardiovascular (CV) side effects in using anastrozole, letrozole, and exemestane based on original studies on AIs vs placebo or tamoxifen were compared. We integrated all available direct and indirect evidences. The odds ratio (OR) of severe CV events for indirect comparisons between exemestane and anastrozole was 1.41 (95% confidence interval [CI] =0.49–2.78), letrozole and anastrozole was 1.80 (95% CI =0.40–3.92), and letrozole and exemestane was 1.46 (95% CI =0.34–3.4). OR of subgroup risk for AIs and tamoxifen were all >1 except for thrombolism risk subgroup. The results showed that the total and severe CV risk ranking is letrozole, exemestane, and anastrozole in descending order. None of the AIs showed advantages in CV events than tamoxifen except for thromboembolism event incidence.
Introduction
Hormonal therapy remains a standard form of therapy in the treatment of endocrine-positive breast cancer. Large-scale clinical trials have proved that 5 years of endocrine therapy significantly reduced the recurrence rate and mortality in adjuvant setting.Citation1–Citation3 The results of trials carried out with the third generation of aromatase inhibitors (AIs) indicated better disease-free survival (DFS) among patients with postmenopausal endocrine-responsive breast cancer than those given tamoxifen in the neoadjuvant,Citation4,Citation5 adjuvant,Citation6,Citation7 and metastaticCitation8 settings. AIs are currently part of the standard treatment for patients, including men, with postmenopausal endocrine-responsive breast cancer. Recently, it has been proved that no difference is noted in antitumor efficacy among these three compounds.Citation9 A significant overall survival benefit was expected comparing AIs with tamoxifen; however, in most published literatures, the effect was not significant in randomized controlled trials (RCTs). Some experts believe that the only limitations in using AIs are their tendency to cause side effects. Potential adverse events, including cardiovascular (CV) side effects, should be considered in long-term management of patients taking AIs. AIs reduce estrogen levels by inhibiting the aromatase enzyme and reducing the level of circulating estrogen; thus, further reduction in estrogen level may potentially increase the risk of developing CV disease. The recent meta-analysis by AydinerCitation9 concludes that there is a greater risk of CV events (odds ratio [OR] =1.20; P=0.030) in AI monotherapy than tamoxifen. We first proceeded to a literature-based network meta-analysis of RCTs to evaluate and compare serious and/or life-threatening CV risk reported comparing different AIs in postmenopausal women.
This systematic review complies with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.Citation10
Materials and methods
The authors advise that ethics approval was not applicable for this study as it is a recombination and statistical analysis upon the published studies, all the data were obtained from published data, and all the studies included in this study had ethics approval.
Search strategy
Our systematic review protocol was compiled and reviewed by the team. We searched PubMed, Embase, CENTRAL, CDSR, and DARE databases using the keywords “aromatase inhibitors”, “anastrozole”, “letrozole”, “exemestane”, “tamoxifen”, “breast neoplasm”, “randomized controlled trial”, and similar terms were cross-searched from RCTs. We complemented searches by perusing the reference lists of previous meta-analyses and set no geographical restrictions. Two investigators (XHZ and LL) independently assessed trials for eligibility and extracted data. The Quality of Reporting of Meta-analyses guidelines has been followed throughout the design, implementation, analysis, and reporting of this meta-analysis. All statistical tests were two-sided.
Inclusion and exclusion criteria
Inclusion criteria were drawn according to Participants, Intervention, Comparison, Outcome, Study design (PICOS)Citation11 approach. RCTs that enrolled postmenopausal patients with hormonal receptor positive were eligible. The intervention is one AI regime including anastrozole, exemestane, letrozole monotherapy, or following tamoxifen, and the control group is tamoxifen in monotherapy or placebo following initial tamoxifen in sequential therapy. The prespecified primary outcome was fatal or nonfatal myocardial infarction. Secondary outcomes were hemorrhagic or ischemic stroke, CV death, death of unknown cause, and death from any cause. We attempted to avoid duplication of information from multiple reports on the same trial by considering only the data from the report containing detailed events with the longest follow-up. The flowchart is shown in . Accordingly, the present meta-analysis incorporates more recent results and covers a larger patient population.
Data extraction
Data abstraction was performed by two independent observers who extracted the data from the respective trials and verified the results by comparison. Data of only severe side effects (3–5 grade or death) were extracted.
Statistical analysis
Whenever possible, we used data from studies with the longest follow-up available. We excluded comparisons with zero events in both groups from the relevant analysis since such comparisons provide no information on the magnitude of the treatment effect. In main analysis, all trials with available quantitative information were utilized. For all calculations, we undertook subgroup analyses according to the type of CV events (myocardial infarction, cerebrovascular events, thromboembolism, CV death, non-breast cancer-related death, and breast cancer-related death). We used a Bayesian random effects model, which fully preserved randomized treatment comparisons within trials. Analysis was done using Markov chain Monte Carlo methods with minimally informative prior distributions. We did separate random effects meta-analyses for all available direct comparisons (head-to-head comparisons of two treatments in the same RCT). The extent was quantified to study heterogeneity with I2 (ranging between 0% and 100%). To check the robustness of our analyses, we calculated Bayesian random effects meta-analysis for all accessible direct comparisons. For all analyses, we used Stata release 12.0 with the metan routine (a Stata routine for fixed and random effects meta-analysis), and WinBUGS version 1.4 (MRC Biostatistics Unit, Cambridge, UK). The difference in ORs derived from direct and indirect comparisons was plotted.
Results
Of the 1,522 studies screened, full text of 23 studies had been assessed and ten trials consisting of a total number of 36,204 patients were included in this meta-analysis. Three studies without suitable design, eight reviews, and two trial publications without cardiac side effect records were excluded. The flow chart is shown in , and characteristics of the included trials are presented in . The network relationship among the five strategies and the number of patients involved are shown in . In addition, the direct comparisons included in this study are represented. There are five interventions in this study (anastrozole, exemestane, letrozole, tamoxifen, and placebo), and the lines connecting them represent the direct comparisons and the number of patients included in this study. According to the number of patients, the thickness of lines varies.
Figure 2 Network relationship diagram.
Abbreviation: RCTs, randomized controlled trials.
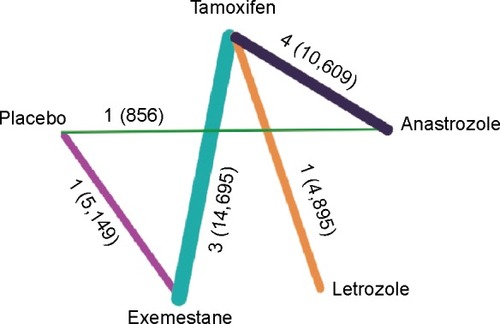
Figure 3 The calculated results of the network meta-analysis.
Abbreviations: AIs, aromatase inhibitors; OR, odds ratio; CV, cardiovascular; CI, confidence interval.
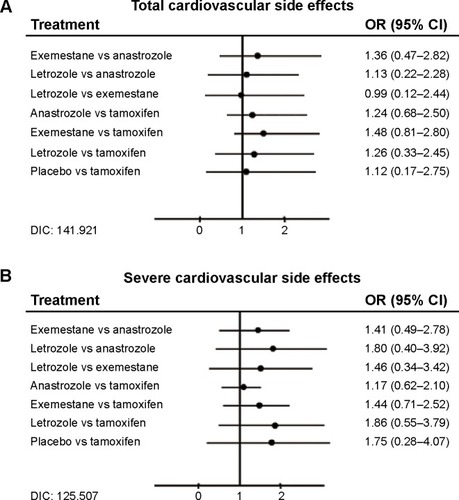
Table 1 The characteristics of the included trials
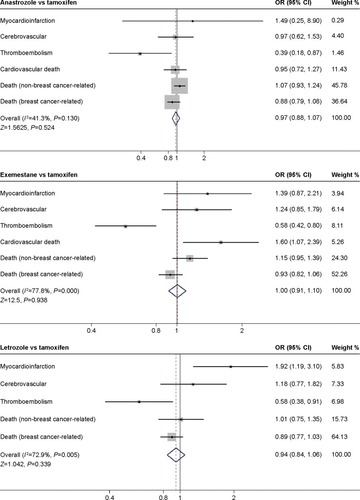
We extracted the data from ten trials and calculated the CV risk incidence. The onset distribution and OR of the direct comparisons are listed in . The OR density diagram is shown in . After 30,000 times of iteration, the Markov Chain Monte Carlo values of each comparison were fluctuated and finally stable at 1, which meant the sampling error was too small to effect the results. In indirect comparisons, the onset distribution of CV risk was not significant (ORs were beyond 95% confidence intervals [CI]) and hence not listed. In direct comparisons of CV side effect, anastrozole (OR =1.0, 95% CI =0.994–1.005, P=0.815) and exemestane (OR =1.007, 95% CI =0.998–0.015, P=0.103) showed no significant effect compared with tamoxifen, whereas the effect of letrozole was less, as only one selected experiment (BIG1-98)Citation12,Citation13 directly compared letrozole and tamoxifen and carried out χ2 test (χ2=13.211, P=0.0003). According to the subgroup analyses, all three AIs showed OR >1 compared with tamoxifen in myocardial infarction (anastrozole: OR =1.489, 95% CI =0.249–8.898; exemestane: OR =1.389, 95% CI =0.871–2.215; letrozole: OR =1.919, 95% CI =1.187–3.102). Among the AIs, anastrozole related with the lowest CV risk generally speaking. Anastrozole showed as superior to tamoxifen in severe CV risk incidence except for myocardial infarction and non-breast cancer related death (OR =1.489, 95% CI =0.249–8.898; OR =1.074, 95% CI =0.933–1.238). The risk of non-breast cancer-related mortality appeared to be increased with anastrozole, exemestane, and letrozole (anastrozole: OR =1.074, 95% CI =0.933–1.238; exemestane: OR =1.151, 95% CI =0.954–1.389; letrozole: OR =1.010, 95% CI =0.754–1.353; ) and the breast cancer-related mortality appeared to be decreased (anastrozole: OR =0.885, 95% CI =0.788–1.081; exemestane: OR =0.932, 95% CI =0.822–1.062; letrozole: OR =0.985, 95% CI =0.774–1.034).
Figure 4 OR density diagram after 30,000 times of iteration, MCMC=1.
Abbreviations: ANA anastrozole; EXE, exemestane; LET, letrozole; MCMC, Markov Chain Monte Carlo; OR, odds ratio; PLA, placebo; TAM, tamoxifen.

Table 2 Characteristics of trials across different direct comparisons
The rank of cardiac side effect is listed in . Among the three AIs, anastrozole was found to be less hazardous. OR value was 1.36 (95% CI =0.47–2.82) compared with exemestane and 1.13 compared with letrozole (95% CI =0.22–2.28). Letrozole and exemestane were almost the same in total CV risk.
All the trials reported severe CV events constituting a total number of 1,004 patients. The onset incidence was 2.02% (305/15,084) in tamoxifen strategy and 3.07% (555/18,074) in AI strategy, just corresponding to a recent report about endocrine treatment side effect.Citation14 According to the result of network meta-analysis, the OR values of AI to tamoxifen were all greater than 1. OR of anastrozole vs tamoxifen was 1.17 (95% CI =0.62–2.10), exemestane vs tamoxifen was 1.44 (95% CI =0.71–2.52), and letrozole vs tamoxifen was 1.86 (95% CI =0.55–3.79). Among the three AIs, letrozole represented a higher OR value than the other two and anastrozole represented the lowest OR (OR of letrozole vs anastrozole =1.80, 95% CI =0.40–3.92; OR of letrozole vs exemestane =1.46, 95% CI =0.34–3.42; OR of exemestane vs anastrozole =1.41, 95% CI =0.49–2.78; ). In the subanalysis of indirect comparison of AIs for each CV disease such as myocardial infarction, the I2s were >50%, which meant the heterogeneities of subgroups were too obvious to analyze.
As for tamoxifen, it showed no more CV risks in subgroup analysis compared with exemestane and letrozole; however, the thromboembolism risk was greater than three AIs (anastrozole vs tamoxifen: OR =0.393, 95% CI =0.178–0.868, P=0.03; exemestane vs tamoxifen: OR =0.579, 95% CI =0.418–0.801, P=0.508; letrozole vs tamoxifen: OR =0.585, 95% CI =0.377–0.907, P=0.508; ).
Figure 5 Forest plot of comparison of cardiovascular side effects between AIs and tamoxifen.
Abbreviations: OR, odds ratio; CI, confidence interval; AIs, aromatase inhibitors.
Discussion
Albeit reduced cancer-related mortality necessitates AI intake, the compliance remains relatively low due to side effects, especially CV events, fractures, and menopausal symptoms.
AIs block estradiol biosynthesis from androgens by inhibiting aromatase, which are expected to induce extensive alterations in human body. Functional estrogen receptors are detected in vascular endothelial cells and smooth muscle cells.Citation15,Citation16 Estrogen receptor β (ERβ) plays a dominant role in protecting myocardial cells from afterload pressure. Similar phenotypes with hypertension cardiac hypertrophy can be seen in ERβ knockout mice. Increase in ERα gene expression can improve the stability of intercalated discs of the myocardial cells.Citation17
Reducing circulating estrogen in plasma can also lead to lipid metabolism interruption. High-density lipoprotein cholesterol (HDL) was likely to decline after 3 months after initiation of AI therapy in women and generally remained stable throughout the studies.Citation17,Citation18 Exemestane can induce androgen-like effects that are still controversial in CV system.Citation19,Citation20 Tamoxifen lowers serum cholesterol after 2 weeks of administration, and this may contribute to cardiac protection.
Theoretically speaking, Letrozole, also called the fourth-generation AI, as well as exemestane demonstrate a better inhibition to aromatase activity. Compared with anastrozole, letrozole and exemestane may represent weaker protection to myocardium due to the strong inhibition of estrogen.
Aydiner conducted a meta-analysis on breast cancer outcome of several adjuvant hormonal therapy regimes. He announced no difference between monotherapy and sequenced therapy in CV risk (OR =1.20 and 1.15; P=0.030 and 0.003, respectively), whereas both of them are of high risk in myocardial disease.Citation21 In the study of Josefsson and Leinster, no differences were observed for CV disease of different regimes.Citation22
Cardiac complications arise from complex interactions of multiple factors. The prime issues can be summarized as preexisting patient factors, cancer-related factors, toxic effects of the drugs, and radiation dose of heart. The snowball effect of the consolidated result will finally turn to increased risk. The incidence of late-onset ventricular dysfunction appears to increase in conjunction with the length of the follow-up.Citation3 An unanswered question is that no data are available regarding the timing of onset. It questions the patient’s vulnerability of long-term AI regime. AIs have a somewhat different adverse-effect profile. Individualized treatment should provide more survival benefits with less serious events considering the biological type, grade of disease, and antecedent history of CV disease.
Till now, far less is known about head-to-head comparison among AIs. Although FACECitation23 and MA.27Citation24 trials are ongoing, cardiac details are still under investigation.
In this network meta-analysis, we found a significant superiority of anastrozole to letrozole and exemestane. The hazard is almost reduced to half when compared with letrozole (). In subgroup analysis, the result was still pronounced. Letrozole is shown to provide lower non-breast cancer-related death (letrozole vs tamoxifen: 35.32‰ vs 34.97‰, anastrozole vs tamoxifen: 71.51‰ vs 67.65‰, exemestane vs tamoxifen: 31.51‰ vs 27.27‰), while anastrozole has decreased rate of causing myocardial infarction, cerebral disease, thromboembolism, and CV death (). It confirms that anastrozole is more suitable for the continuous endocrine therapy for longer duration when basal CV disease exists.
Network meta-analysis not only increases statistical power by incorporating evidence from both direct (head-to-head) and indirect comparisons across all five interventions but can also provide insights into the relative effectiveness of interventions that have never been directly compared, such as anastrozole therapy and letrozole therapy. It combines direct and indirect evidences on the relative effectiveness of several interventions with respect to randomization. An important feature of this methodology is that heterogeneity between trials is set to zero. Thus, the underlying true treatment effects are assumed homogeneous. Network meta-analysis concerns more about the fitness of models and model consistency than the heterogeneity of the data. In this analysis, the heterogeneity is difficult to avoid because the drug administration regimes are not same, and they can include monotherapy and sequenced therapy,Citation25–Citation28 or even extended therapy.Citation29,Citation30 The population in each trial differs and the average age differs in trials. In the network diagram, we can see that the number of people assigned in exemestane trial are 14,695, which is >10,609 in anastrozole and 4,895 in letrozole; thus, data in the analysis can be biased. In data selection and processing, for example in BIG-198, we chose patients getting monotherapy rather than sequenced therapy groups among the four groups. In ABCSG 6a,Citation29 although the result is the comparison between placebo and anastrozole, it indeed represents tamoxifen for 5 years compared with tamoxifen for 5 years followed by anastrozole for 3 years. As for patients’ age, trials of anastrozole (ABCSG-6a and ATAC) included more elderly patients (average age =67.8 and median age =65, respectively), which contributed to non-breast cancer-related deaths. Despite the fact that random effect model was chosen to reduce the effect caused by heterogeneity, the effect is difficult to eliminate. In some subgroup analysis, the data cannot be analyzed due to the excessive heterogeneity.
This analysis is the first network meta-analysis about comparison of three AIs on CV toxicity. Experts had done lot of studies on direct comparison between AI and tamoxifen, while there is no direct evidence about head-to-head comparison between AIs. In this article, indirect comparison will provide some guidance for patients’ choices on drug. This study has certain limitations. First, for the ATAC study, the earlier published editionCitation31 rather than the latter one reported the detailed CV events, although the latter one had a longer follow-up.Citation3 In ABCSG-6a trial, only myocardial infarction rate was recorded.Citation32 In TEAM trial,Citation33 some patients were not graded. This may have influence on the statistical result. Second, the criteria of CV toxicity in different trials may be different, and the patient’s baseline varied among trials. The results of the present meta-analysis should be cautiously interpreted in addition to the risk of publication bias that exists in any meta-analysis. Third, in network analysis, results calculated through WinBUGs are represented as OR value without P-value; thus, it is difficult to explain the significance of differences between three AIs.
Implications and conclusion
From our study, anastrozole was found to be less toxic compared with exemestane and letrozole, while letrozole was found to be the most toxic. Similar to previous reports, AIs are associated with more CV risk than tamoxifen. In the treatment with anastrozole and exemestane, the risk of non-breast cancer-related mortality appeared to increase (Letrozole showed almost the same effect with tamoxifen), while the breast cancer-related mortality appeared to decrease. Ultimately, AI represents the standard adjuvant endocrine regime for postmenopausal women with endocrine-responsive disease. Because of the reduction of estrogen, avoiding the side effects is difficult. Their benefit appeared to be always balanced with a potential increase in non-breast cancer-related hazard, especially in long-term follow-up. It is wise and necessary to select an appropriate endocrine therapy drug and make specific periodic examination according to an individual’s condition and underlying disease.
Acknowledgments
This work is supported by a grant from the Natural Science Foundation of China (number 81472806).
Disclosure
The authors report no conflicts of interest in this work.
References
- LinNUWinerEPAdvances in adjuvant endocrine therapy for postmenopausal womenJ Clin Oncol200826579880518258989
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet200536594721687171715894097
- CuzickJSestakIBaumMATAC/LATTE investigatorsEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trialLancet Oncol201011121135114121087898
- SmithIEDowsettMEbbsSRIMPACT Trialists GroupNeoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: the Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trialJ Clin Oncol200523225108511615998903
- CataliottiLBuzdarAUNoguchiSComparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: the Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trialCancer2006106102095210316598749
- Arimidex, Tamoxifen, Alone or in Combination (ATAC) Trialists’ GroupForbesJFCuzickJEffect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trialLancet Oncol200891455318083636
- KudachadkarRO’ReganRMAromatase inhibitors as adjuvant therapy for postmenopausal patients with early stage breast cancerCA Cancer J Clin200555314516315890638
- ThürlimannBHessDKöberleDAnastrozole (‘Arimidex’) versus tamoxifen as first-line therapy in postmenopausal women with advanced breast cancer: results of the double-blind cross-over SAKK trial 21/95 – a sub-study of the TARGET (Tamoxifen or ‘Arimidex’ Randomized Group Efficacy and Tolerability) trialBreast Cancer Res Treat200485324725415111763
- AydinerAMeta-analysis of breast cancer outcome and toxicity in adjuvant trials of aromatase inhibitors in postmenopausal womenBreast201322212112923462682
- AmirESerugaBNiraulaSCarlssonLOcanaAToxicity of adjuvant endocrine therapy in postmenopausal breast cancer patients: a systematic review and meta-analysisJ Natl Cancer Inst2011103171299130921743022
- CostantinoGMontanoNCasazzaGWhen should we change our clinical practice based on the results of a clinical study? Searching for evidence: PICOS and PubMedIntern Emerg Med201510452552725833085
- ReganMMNevenPGiobbie-HurderABIG 1-98 Collaborative Group, International Breast Cancer Study Group (IBCSG)Assessment of letrozole and tamoxifen alone and in sequence for postmenopausal women with steroid hormone receptor-positive breast cancer: the BIG 1-98 randomised clinical trial at 8.1 years median follow-upLancet Oncol201112121101110822018631
- ColleoniMGiobbie-HurderAReganMMAnalyses adjusting for selective crossover show improved overall survival with adjuvant letrozole compared with tamoxifen in the BIG 1-98 studyJ Clin Oncol20112991117112421321298
- HoppTAWeissHLParraISCuiYOsborneCKFuquaSALow levels of estrogen receptor beta protein predict resistance to tamoxifen therapy in breast cancerClin Cancer Res200410227490749915569979
- KarasRHPattersonBLMendelsohnMEHuman vascular smooth muscle cells contain functional estrogen receptorCirculation1994895194319508181116
- VenkovCDRankinABVaughanDEIdentification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial functionCirculation19969447277338772695
- BellLNNguyenATLiLConsortium on Breast Cancer Pharmacogenomics (COBRA)Comparison of changes in the lipid profile of postmenopausal women with early stage breast cancer treated with exemestane or letrozoleJ Clin Pharmacol201252121852186022174434
- YounusMKissnerMReichLWallisNPutting the cardiovascular safety of aromatase inhibitors in patients with early breast cancer into perspective: a systematic review of the literatureDrug Safety201134121125114922077502
- McCrohonJAJessupWHandelsmanDJCelermajerDSAndrogen exposure increases human monocyte adhesion to vascular endothelium and endothelial cell expression of vascular cell adhesion molecule-1Circulation199999172317232210226099
- MorrisonJASprecherDLBiroFMApperson-HansenCDipaolaLMSerum testosterone associates with lower high-density lipoprotein cholesterol in black and white males, 10 to 15 years of age, through lowered apolipoprotein AI and AII concentrationsMetabolism200251443243711912549
- JosefssonMLLeinsterSJAromatase inhibitors versus tamoxifen as adjuvant hormonal therapy for oestrogen sensitive early breast cancer in post-menopausal women: meta-analyses of monotherapy, sequenced therapy and extended therapyBreast2010192768320096578
- MouridsenHKeshaviahACoatesASCardiovascular adverse events during adjuvant endocrine therapy for early breast cancer using letrozole or tamoxifen: safety analysis of BIG 1-98 trialJ Clin Oncol200725365715572217998546
- O’ShaughnessyJA decade of letrozole: FACEBreast Cancer Res Treat2007105suppl 1677417912637
- GossPEIngleJNPritchardKIExemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27 – a randomized controlled phase III trialJ Clin Oncol201331111398140423358971
- JakeszRJonatWGnantMABCSG and the GABGSwitching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trialLancet2005366948445546216084253
- BoccardoFRubagottiAGuglielminiPSwitching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trialAnn Oncol200617suppl 7vii10vii1416760270
- AiharaTTakatsukaYOhsumiSPhase III randomized adjuvant study of tamoxifen alone versus sequential tamoxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 studyBreast Cancer Res Treat2010121237938720390343
- BertelliGHallEIrelandELong-term endometrial effects in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES) – a randomised controlled trial of exemestane versus continued tamoxifen after 2–3 years tamoxifenAnn Oncol201021349850519717534
- JakeszRGreilRGnantMAustrian Breast and Colorectal Cancer Study GroupExtended adjuvant therapy with anastrozole among postmenopausal breast cancer patients: results from the randomized Austrian Breast and Colorectal Cancer Study Group trial 6aJ Natl Cancer Inst200799241845185318073378
- GossPEIngleJNMartinoSRandomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17J Natl Cancer Inst200597171262127116145047
- Arimidex, Tamoxifen, Alone or in Combination Trialists’ GroupBuzdarAHowellAComprehensive side-effect profile of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: long-term safety analysis of the ATAC trialLancet Oncol20067863364316887480
- MamounasEPJeongJHWickerhamDLBenefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trialJ Clin Oncol200826121965197118332472
- van de VeldeCJReaDSeynaeveCAdjuvant tamoxifen and exemestane in early breast cancer (TEAM): a randomised phase 3 trialLancet2011377976232133121247627
- ParidaensRDirixLLohrischCEuropean Organization for the Research and Treatment of Cancer (EORTC)- Investigational Drug Branch for Breast Cancer (IDBBC)Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancerAnn Oncol20031491391139812954578