Abstract
Immuno checkpoint inhibitors have ushered in a new era with respect to the treatment of advanced non-small-cell lung cancer. Many patients are not suitable for treatment with epidermal growth factor receptor tyrosine kinase inhibitors (eg, gefitinib, erlotinib, and afatinib) or with anaplastic lymphoma kinase inhibitors (eg, crizotinib and ceritinib). As a result, anti-PD-1/PD-L1 and CTLA-4 inhibitors may play a novel role in the improvement of outcomes in a metastatic setting. The regulation of immune surveillance, immunoediting, and immunoescape mechanisms may play an interesting role in this regard either alone or in combination with current drugs. Here, we discuss advances in immunotherapy for the treatment of metastatic non-small-cell lung cancer as well as future perspectives within this framework.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction to the development of immunotherapy for cancer and NSCLC
According to the World Health Organization, lung cancer is currently the leading cause of cancer mortality worldwide and of tobacco-related death.Citation1–Citation3 The 5-year overall survival (OS) rate is only 15% for all stages.Citation4 These results also revealed that invasion and metastasis are the primary causes of recurrence and death in patients with lung cancer.Citation4 Historical approaches to nonspecific cytotoxic chemotherapy are associated with severe adverse effects (AEs), selection of drug-resistant tumor cells (TCs), and failure to resolve metastatic or subclinical disease.Citation3
Lung cancer can be generally divided into small-cell lung cancer and non-small-cell lung cancer (NSCLC). NSCLC accounts for >80% of all lung cancers, and patients with this type demonstrate a limited response to chemotherapy when they are in advanced stages.Citation5 Approximately 75% of all NSCLC cases are diagnosed at an advanced stage of the disease,Citation6,Citation7 which means that these patients will have a median survival time of 4–5 months after diagnosis, and only 10% of them will survive for 1 year.Citation8,Citation9
Despite this scenario, the search for safe and specific NSCLC treatments found an opportunity in various immunotherapeutic agents. Vaccines, cytokines, and monoclonal antibodies (mAbs) have become promising drugs that may either help to generate an active immune response against neoplastic antigens or stimulate a nonspecific immune attack against various tumors; in addition, external antibodies may be used as a mechanism for a brief targeted response.Citation2 Increased knowledge of the intricacies of the immune system and how it might act in synergy with conventional chemotherapy has generated new perspectives for NSCLC therapy, which may change its prognosis.
In recent years, many attempts have been made to obtain consistent benefits from tumor vaccines and cytokines: belagenpumatucel-L (an allogeneic TC vaccine) and tecemotide (a peptide vaccine) did not meet survival end points over the placebo in Phase III trials.Citation10,Citation11 The use of IL-2 and interferons (cytokines) generated infrequent responses, and these were observed in only a few types of cancers. Researchers question whether this limited response is actually caused by a tumor mechanism of immune escape.Citation12
The last drug class, mAbs, became applicable as part of a very specific strategy: not as a direct immune system activator against cancerous cells but rather as an instrument to free T-cells from negative regulatory breaks and to promote their cytotoxicity so that they may bind to distinct sites.Citation12 Research has revealed that the targeted proteins that are responsible for such negative regulations are PD-1, PD-L1, and CTLA-4. Ipilimumab, pembrolizumab, tremelimumab, and nivolumab are all immune checkpoint inhibitors that are capable of binding and inactivating the effects of the above-mentioned proteins. These drugs have demonstrated positive results in the treatment of many cancers and have been studied for use in NSCLC. The US Food and Drug Administration (FDA) has recently approved nivolumab for therapeutic use.Citation13
This review aims to discuss the role and evidence of immune response in cancer pathology, the mechanisms of immune checkpoint inhibitors, clinical trials and their results with respect to efficacy and safety, and future perspectives for patients’ quality of life (QoL).
Rationale for the development of immunotherapies
The immune system functions as an adaptable and specific system that distinguishes self from nonself and attacks foreign pathogens and infected self tissues. The innate immune system acts as a nonspecific first line of defense and includes a vast array of components, including antigen-presenting cells (APCs). In contrast, the adaptive immune response results in the development of cytotoxic CD8+ T-cells, helper CD4+ T-cells, and antibody-producing plasma cells.Citation14,Citation15 The presence of an adaptive immune system endows vertebrates with a unique ability to develop highly specific responses. The adaptive immune system is driven by a multitude of highly specific antigen receptors on T-cells (T-cell receptor) and B-cells (B-cell receptor). The cognate binding of an antigen to the B- or T-cell receptor promotes the development of a vigorous antigen-specific immune response and the development of long-lived memory cells. After the eradication of cancer, the presence of memory cells potentially prevents tumor regrowth, decreases metastatic spread, and can limit the de novo induction of a second malignancy.Citation8
In antitumor immune responses, CD8+ T-cells and CD4+ T-cells recognize tumor antigens in the context of major histocompatibility complex class I and class II molecules, respectively. Following initial APC-driven activation, CD8+ T-cells function in cell-mediated cytotoxicity and have the ability to kill cells that are recognized as nonself and cells with altered self-antigens. It is thought that CD8+ T-cells play a key role in the antitumor immune response. On the contrary, CD4+ T-cells differentiate into several types of helper CD4+ T-cells. In a noninflamed environment, CD4+ T-cells can differentiate into regulatory T-cells (Tregs). These cells are able to inhibit the host’s antitumor immune response as they are important negative regulators of the immune system. When this is taken into account, Tregs are considered as a good target for cancer immunotherapy.Citation8
Published data on animal studies have revealed that lymphocytes and natural killer cells contribute to host antitumor defense mechanisms. These data show that deficiencies in key immunologic molecules, such as the perforin,Citation7 RAG2,Citation16 or IFN-γ,Citation17 lead to the development of spontaneous tumors. These studies clarify the cellular basis of cancer immunosurveillance, which is a hypothesis that was proposed decades ago by BurnetCitation18 and Thomas.Citation19
A clear difference in the interpretations of Burnet and Thomas in regard to this hypothesis was the nature of tumor recognition by immune cells (ICs). Burnet considered the self-versus nonself-discrimination hypothesis in which the development of cancers is inhibited. The hypothesis devised by Thomas was different in that he proposed that organisms must possess a primary defense, similar to homograft rejection, against neoplasia.Citation4 The immune surveillance theory, which was validated after technological advances in mouse genetics and mAb production, involves a set of cells and immune system molecules that play a role in the active elimination of immunogenic TCs.
However, many have acknowledged that cancer immunosurveillance is just one step in a larger process termed cancer immunoeditingCitation9,Citation20,Citation21 (). This concept recognizes that even after the phase of elimination when the tumor escapes immunosurveillance, the fate of the tumor may ultimately be sculpted by immunity and may experience two subsequent phases, as follows: the equilibrium phase, during which the tumor may either be dormant or be immunologically sculpted by immune “editors” to produce new variants that carry more mutations, which would increase resistance to immune attack; this phase would be followed by the escape phase, when the tumor becomes clinically detectable. The elimination of the tumor or the long-term control of cancer in equilibrium represents a potential goal for immunotherapy.Citation21
Figure 1 Immunoediting mechanism.
Abbreviations: DC, dendritic cell; M0, macrophage; NK, natural killer cell; Treg, regulatory T-cell; MDSC, myeloid-derived suppressor cell.
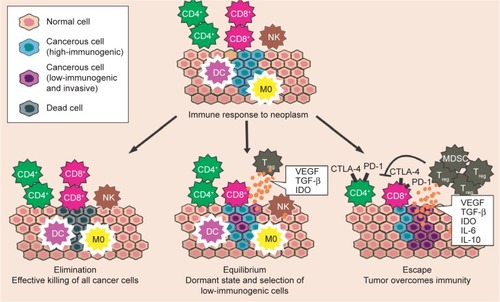
The immune system is suppressed by several factors. It would be a great challenge if the elaboration of immunotherapeutic strategies could determine which immunosuppressive factors are required for the maintenance of immune tolerance to various types of cancers.Citation22 The immune responses can be inhibited when certain TCs overproduce and express several molecules with proapoptotic or immune suppressive properties, such as TGF-β; this protein constitutes one of the most important immunosuppressive factors because it inhibits the activation, proliferation, and activity of lymphocytes. In addition, IL-10, which is an anti-inflammatory or immunosuppressive cytokine, is associated with tumor growth and with the regulation of the maturation of APCs as well as their capacity to produce inflammatory cytokines, such as IL-12.Citation23
The number or frequency of tumor-infiltrating lymphocytes is usually used as a prognostic factor, where an increase in tumor-infiltrating lymphocytes is usually a marker of a good prognosis and is associated with prolonged survival of cancer patients.Citation24,Citation25 Nevertheless, the protective functions of the immune system, in which normal conditions are essential to induce immune tolerance to self-antigens, may also provide the means for tumor escape. Thymus-derived or peripherally induced Foxp3+ Tregs act to inhibit autoimmune responses, but in the tumor microenvironment, these cells have the ability to suppress the tumor-specific T-cell response via the production of immunosuppressive cytokines (IL-10 and TGF-β) and via the expression of negative co-stimulatory molecules (CTLA-4, PD-1, and PD-L1). An increased number of Foxp3+ Tregs has been found in the blood of patients with cancer.Citation26 Myeloid-derived suppressor cells (MDSCs) play key roles in the inhibition of host-protective antitumor responses via the induction of Tregs.Citation9 In addition, IDO, which is an enzyme involved in the tryptophan catabolism pathway, may elicit the suppression of T and natural killer cells, the generation and activation of Tregs and MDSCs, and the promotion of tumor angiogenesis. IDO is also overexpressed in TCs.Citation27
The TCs can also induce an upregulation of CTLA-4 for their own advantage. The CTLA-4/B7 engagement inhibits lymphocyte activation and proliferation, which may alter the immune response and promote tumor escape. The interaction of PD-1 and its ligand has also been described to negatively regulate the proliferation and cytokine production of T-cells. The ability of TCs to induce a hostile microenvironment through immunosuppression is a significant barrier to effective cell-mediated immunity and immunotherapy.Citation26
Mechanism of action of emerging immunotherapies such as PD-1 inhibitors
Mechanisms that are used by cancer cells to evade the host immune response are valuable targets for immunotherapy. The loss of antigen expression and resistance to cytotoxicity are the two ways that TCs overcome immunity. Most likely, cells with those advantages experience random mutations and natural selection because T-cells or phagocytes do not easily destroy them. Cancer cells, however, can also change their microenvironment to an immunosuppressive state via cytokine expression (TGF-β, VEGF, or IDO) (). In addition, through the recruitment of Tregs (a subtype of helper T-cells) and MDSCs, TCs can actively induce a state of immunosuppression. Thus, with the ultimate goal of reshaping the tumor microenvironment, several immunotherapeutic strategies that inhibit tumor-induced immunosuppression have been developed. Specifically, the use of mAbs directed at PD-1, PD-L1, and CTLA-4 has shown promising results.Citation28
CTLA-4 was the first immune checkpoint receptor to be a clinical target. This protein is normally expressed on the plasma membranes of Tregs and memory T-cells. There, it downregulates T-cell activation by competing with CD28 for its ligands, CD80 and CD86, and by inducing cell cycle arrest in T-cells.Citation29 CTLA-4, whose action occurs 24–48 hours after antigen presentation, primarily regulates CD4+ T-cells and enhances the immunosuppressive activity of Tregs.Citation30 PD-1 allows for immune resistance because it leads to the inhibition of effector T-cell activity when it binds to ligands such as PD-L1, which is a protein expressed by tumor and stromal Tregs. Similar to CTLA-4, PD-L1 can also compete with CD28. Because cancer cells induce a microenvironment that is highly populated by Tregs, they are very dependent on CTLA-4, PD-1, and PD-L1 activities to promote immunosuppression.Citation31 Ipilimumab and tremelimumab are mAbs that were developed to inhibit CTLA-4. Nivolumab, pembrolizumab, atezolizumab, and durvalumab were developed to inhibit PD-1 and PD-L1. Through a reduction in immune checkpoint activity in a Treg-rich microenvironment, they are able to diminish tumor evasion ().Citation32
Figure 2 Mechanism of action of immune checkpoint inhibitors.
Abbreviations: Tregs, regulatory T-cells; TCR, T-cell receptor; MHC, major histocompatibility complex.
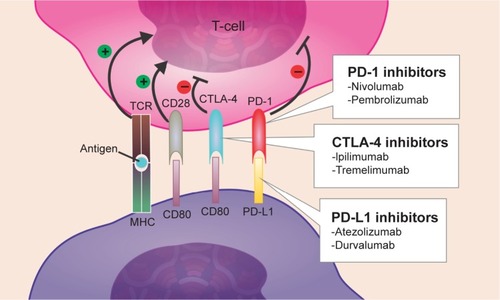
Currently, preclinical studies of CTLA-4 and PD-1 showed that the inhibition of either of these pathways stimulates an antitumor immune response, which increases their validity as therapeutic targets. Furthermore, other studies based on different trials have shown that patients whose tumors overexpress PD-L1 tend to demonstrate an increased response to anti-PD-L1-directed therapy.Citation33
Efficacy and safety of immunotherapy for NSCLC
A blockade of immune checkpoints, including the inhibition of the CTLA-4 and PD-1/PD-L1 pathways, has introduced a new era in cancer treatment, as advances in intratumoral immune responses have been observed in numerous preclinical studies.Citation34 This section features the major results of Phase I–III trials that primarily involve the efficacy and safety of the use of mAbs in NSCLC, as well as studies of a combination of these immunotherapies ().
Table 1 Efficacy and safety of immunotherapies for NSCLC
Anti-PD-1 mAb inhibitors
Nivolumab
Nivolumab is a fully humanized IgG4 mAb that targets PD-1. This mAb has shown anticancer activity against several tumor types including NSCLC.Citation35 The first Phase I clinical trial of an anti-PD-1 antibody showed activity in NSCLC, and subsequent studies have demonstrated that a PD-1 pathway blockade supports durable tumor responses.Citation36 Completed Phase I and II clinical trials have recently led to the FDA approval of second-line chemotherapy treatment for resistant squamous NSCLC.Citation37
In a Phase I study of ~300 patients with advanced solid tumors, 22 (17%) of 129 patients with NSCLC achieved an interesting objective response after nivolumab treatment. Treatment with nivolumab also resulted in an OS rate of 42% (95% confidence interval [CI] 33–50) at 1 year, 24% (95% CI 17–33) at 2 years, and 18% (95% CI 11–25) at 3 years; similar results have been reported for the nonsquamous and squamous histological subtypes.Citation35 In another Phase I study that included 122 patients with NSCLC, nivolumab was administered once every 2 weeks at doses of 0.1–10 mg/kg. The results of this study showed that the objective response rate (ORR) across all doses was 18% (95% CI 11–29). Previously, patients were highly treated (54% received more than three lines of chemotherapy), and responses were seen across all dose levels; however, the ORR was longer for the 3 mg/kg dose (32%).Citation3
The safety of nivolumab in patients with NSCLC, specifically in those with squamous cell cancer, was established in a Phase II trial (CHECKMATE-063) in patients who had progressed after systemic chemotherapy. The most common AEs were fatigue (50%), dyspnea (38%), musculoskeletal pain (36%), decreased appetite (35%), cough (32%), nausea (29%), and constipation (24%). Serious AEs occurred in 59% of patients and included dyspnea, pneumonia, exacerbation of COPD, pneumonitis, pleural effusion, and hemoptysis. These adverse reactions led to the discontinuation of treatment in 27% of patients.Citation38
One randomized trial, in which patients received mono-therapeutic nivolumab (3 mg/kg every 2 weeks, intravenously) or docetaxel (75 mg/m2 every 3 weeks, intravenously), showed that the median OS was higher in patients in the nivolumab group than in the docetaxel group (9.2 versus 6.0 months) (95% CI 7.3–13.3) (hazard ratio [HR] 0.59, 95% CI 0.44–0.79; P<0.001). At 1 year, the OS rate was 42% (95% CI 34–50) in the nivolumab group and 24% (95% CI 17–31) in the docetaxel group. Nine (7%) out of 131 patients in the nivolumab group and 71 (55%) out of 129 in the docetaxel group had grade 3/4 treatment-related adverse events.Citation39
The successful use of nivolumab as a second-line therapy has led to studies of nivolumab as a first-line treatment. Currently, nivolumab is being studied in a Phase I trial to assess its safety and tolerability as a first-line combination therapy or monotherapy in chemotherapy-naïve patients.Citation40
Pembrolizumab
Pembrolizumab is a humanized anti-PD-1 mAb of the IgG4 kappa isotype that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2.Citation38 The results of the KEYNOTE-010 trial support the recent approval of pembrolizumab for the management of advanced NSCLC.Citation41
Based on a Phase I clinical trial of 495 patients who received pembrolizumab, fatigue, pruritus, and decreased appetite are its most common AEs. A clear difference based on the dose or schedule was demonstrated.Citation42 In terms of safety, another study showed that grade 3/4 AEs occurred in only 6% of this population. Recently, the use of pembrolizumab (10 mg/kg every 2 or 3 weeks) showed an ORR of 36% in patients with advanced NSCLC.Citation43
In terms of efficacy, a large randomized Phase II/III study was performed at 202 academic medical centers in 24 countries and involved 1,034 patients with previously treated NSCLC. The tumors of these patients expressed PD-L1 on at least 1% of TCs. Patients in this trial demonstrated that the median OS was 10.4 months with 2 mg/kg of pembrolizumab, 12.7 months with 10 mg/kg of pembrolizumab, and 8.5 months with docetaxel. The OS was longer for 2 mg/kg pembrolizumab versus docetaxel (HR 0.71, 95% CI 0.58–0.88; P=0.0008) and for 10 mg/kg pembrolizumab versus docetaxel (HR 0.61, 95% CI 0.49–0.75; P=0.004). Furthermore, among patients with at least 50% of TCs that expressed PD-L1, the OS was significantly longer with 2 mg/kg of pembrolizumab than with docetaxel (14.9 versus 8.2 months; HR 0.54, 95% CI 0.38–0.77; P=0.0002) and with 10 mg/kg of pembrolizumab than with docetaxel (17.3 versus 8.2 months; HR 0.50, 95% CI 0.36–0.70; P<0.0001).Citation44
Anti-PD-L1 mAb inhibitors
Atezolizumab
Atezolizumab (MPDL3280A) is an engineered human IgG1 mAb against PD-L1.Citation3 A Phase I study investigated the use of the atezolizumab in pretreated advanced cancer patients at doses between 1 and 20 mg/kg given three times per week. Approximately, 23 patients with advanced NSCLC were studied for safety and 41 for efficacy and response. As a result, the ORR and 6-month progression-free survival (PFS) rate were 21% and 45%, respectively. No maximum tolerated dose or dose-limiting toxicities were reported, and there were no cases of grade ≥3 pneumonitis.Citation45
According to preliminary data from a Phase I expansion study in 37 patients with evaluable NSCLC, four of five (80%) with strong PD-L1 expression found by immunohistochemistry responded to atezolizumab versus only four of 28 patients (14%) who were PD-L1-negative. These results were confirmed in a later analysis of the full dataset of 53 patients with NSCLC. High PD-L1 expression on tumor-infiltrating ICs was significantly correlated with response to atezolizumab (P=0.0015), while high PD-L1 expression by TCs was not correlated with response to this drug (P=0.920). The ORR in patients with the highest level of PD-L1 expression on ICs was 83%, while the ORR in patients with high tumor PD-L1 expression was only 38%. On the contrary, the response rates were 14%–20% in patients with no or low PD-L1 expression, respectively.Citation46
The single-group Phase II trial BIRCH showed that atezolizumab monotherapy (1,200 mg, intravenously, every 3 weeks until disease progression, unacceptable toxicity, or loss of clinical benefit) demonstrated efficacy in 659 assessable patients with PD-L1-selected stage IIIB/IV or recurrent NSCLC without active central nervous system metastases.Citation47 The population (24%–27%) that achieved an objective response comprised patients who showed the highest expression of PD-L1 on TCs or ICs.Citation17 Another randomized Phase II trial, POPLAR, included patients with nonsquamous or squamous NSCLC with disease progression after platinum treatment. In this trial, 144 patients received atezolizumab monotherapy (1,200 mg, intravenously, every 3 weeks), and 143 received docetaxel (75 mg/m2, intravenously, every 3 weeks). The results showed that in the intention-to-treat population, the median OS was longer in the atezolizumab group compared with the docetaxel group (12.6 versus 9.7 months; HR 0.73, 95% CI 0.53–0.99; P=0.040). In addition, patients with the highest PD-L1 expression on their TCs or ICs showed the largest improvement. Unexpected AEs did not occur in either study. The results from both studies indicate that the selection of patients according to PD-L1 expression could enable the identification of those who are likely to benefit from atezolizumab treatment.Citation47 Currently, the aim of several ongoing trials is to evaluate atezolizumab in pretreated advanced NSCLC (NCT01846416) and the use of this drug in a first-line setting (NCT02409342, NCT02367781, and NCT02367794).Citation3
Durvalumab
Durvalumab (MEDI4736) is a human IgG1 anti-PD-L1 antibody that has shown a satisfactory safety profile and antitumor activity.Citation48 Currently, there is an ongoing placebo-controlled durvalumab trial (called PACIFIC) in patients with stage III unresectable NSCLC following definitive chemoradiation; other studies are likely to be conducted in the future.Citation49
Anti-CTLA-L4 mAb inhibitors
Ipilimumab
Ipilimumab is a fully humanized IgG1 anti-CTLA-4 mAb that blocks the binding of CTLA-4 to its ligand. In terms of efficacy, a randomized Phase II clinical trial assessed treatment with paclitaxel and carboplatin with or without ipilimumab in treatment-naïve stage IV NSCLC patients. The patients showed improvement in immune-related PFS when ipilimumab was administered after chemotherapy (5.7 versus 4.6 months; P=0.05).Citation34 According to a subset analysis, the immune-related PFS in the phased cohort was longer in patients with squamous histology (HR 0.55) than in patients with nonsquamous histology (HR 0.82, 95% CI 0.52–1.28).Citation36
A recent report that pooled data from clinical trials of ipilimumab showed that ~20% of patients will have a long-term survival of at least 3 years after ipilimumab therapy and that the longest reported survival reached 10 years.Citation29
In terms of safety, grade 3/4 AEs occurred with similar frequency across the different arms (control, 37%; concurrent, 41%; phased, 39%), although grade 4 events appeared more frequently in the ipilimumab arms. Among a total of 204 patients, serious immune-related events, including rash (4%), colitis (10%), and hypophysitis (one case), occurred with similar frequency as in previous studies of ipilimumab.Citation50
Currently, an ongoing Phase III clinical trial is testing whether ipilimumab plus paclitaxel and carboplatin will extend the lives of patients with squamous NSCLC more than placebo plus paclitaxel and carboplatin (ClinicalTrials.gov identifier: NCT01285609).
Tremelimumab
Tremelimumab is a fully humanized IgG2 mAb to CTLA-4.Citation22 One randomized Phase II trial involved 87 advanced NSCLC patients with stable disease who responded after four cycles of first-line platinum-based chemotherapy. The patients were randomized between tremelimumab (15 mg/kg) and best supportive care (BSC). As a result, the rate of PFS at 3 months was similar in each arm, with 20% (90% CI 11.4–33.7) and 14.3% (90% CI 6.4–26.3) in the tremelimumab and BSC arms, respectively.Citation32
In terms of safety, the incidence of grade 3/4 AEs was 20.5% in the tremelimumab group (n=44) and 0% in the BSC group (n=43); the most common grade 3/4 AEs due to the use of tremelimumab were diarrhea and colitis (9.1%).Citation51
Combining anti-PD-1, anti-PD-L1, and anti-CTLA-4 therapies
New studies of novel approaches that incorporate checkpoint inhibitors have shown promising preliminary results, and durable responses have been obtained in patients with NSCLC. The combination of two immunotherapies that target a variety of signaling pathways has added an additional treatment modality to immuno-oncology.Citation34
A completed study in mice demonstrated that, compared with single checkpoint inhibition, double blockade promoted tumor repression in 67% (two-thirds) of mice.Citation25 Some current studies are focused on the combination of checkpoint inhibitors; for example, CA209-012 is an ongoing Phase I trial that aims to compare nivolumab as a monotherapy or combined with chemotherapy, targeted therapy, or ipilimumab in patients with NSCLC (ClinicalTrials.gov identifier: NCT01454102).
The results from a Phase I study of the combination of nivolumab and ipilimumab were recently presented. This study divided the patients into two groups: the first arm received 1 mg/kg of nivolumab plus 3 mg/kg of ipilimumab, while the second arm was given 3 mg/kg of nivolumab plus 1 mg/kg of ipilimumab for four cycles. Both arms then received 3 mg/kg of nivolumab until disease progression or unacceptable toxicity occurred.Citation25 In terms of efficacy, the ORR was 11% and 13% for patients in the first arm with squamous and nonsquamous histology, respectively, and was 33% and 13% for the corresponding groups of patients in the second arm. The ORR, in turn, was higher in the nivolumab (3 mg/kg) and ipilimumab (1 mg/kg) treatment arm. After a comparison of the PFS at 24 weeks, the results showed ORRs of 41% versus 29% (the first arm versus the second arm). Grade 3/4 AEs were reported in 49% of patients across both arms. An analysis showed that the most common severe AEs were pneumonitis, diarrhea, colitis, elevated advanced solid tumors, and ALT enzymes. Three of the 49 patients died due to drug-related toxicities.Citation25
Another Phase I trial that assessed the combination of immunotherapies was performed by Rizvi et al and showed that durvalumab (20 mg/kg every 2 or 4 weeks) plus tremelimumab (1 mg/kg every 4 weeks) has a manageable tolerability profile and antitumor activity in patients with NSCLC. Despite the benefit of anti-PD-1/PD-L1 monotherapy, the combination of durvalumab plus tremelimumab appears to be effective regardless of PD-L1 status, even in patients with no PD-L1 staining in the TC membrane; this is a setting where patients would not be expected to derive significant benefit from anti-PD-1/PD-L1 monotherapy over the current standard of care.Citation48
Currently, other clinical trials are ongoing and involve different combinations of immunotherapies. Tremelimumab is being studied in combination with durvalumab (MEDI4376) for advanced NSCLC (ClinicalTrials.gov identifier: NCT2000947, NCT02453282, and NCT02352948). Other studies of ipilimumab plus pembrolizumab in KEYNOTE-021 and of ipilimumab plus atezolizumab are being pursued (ClinicalTrials.gov identifier: NCT02039674 and NCT02174172, respectively).
Chimeric antigen receptor T-cells
Targeted immunotherapies using chimeric antigen receptors (CARs) to redirect and reprogram patient T-cells have shown promising results in the treatment of acute lymphoblastic leukemia and non-Hodgkin’s lymphoma. CARs are genetically engineered synthetic receptors that endow T-cells with the ability to target specific tumor surface antigens. Second-generation CARs, which combine the activation and co-stimulatory signaling domains, have enabled the design of more potent T-cells that can mediate complete responses in patients with chemo-refractory B-cell malignancies. Although the therapeutic potential of CAR T-cells against solid cancers remains unknown, a recent study has indicated the therapeutic potential of regional CAR T-cell therapy for pleural malignancies (both primary and metastatic). Through CAR T-cells that are specific to mesothelin, a cell surface molecule that is overexpressed in >90% of epithelioid malignant pleural mesotheliomas, the authors show that early antigen activation of mesothelin by CD4+ CAR T-cells can lead to enhanced antitumor efficacy.Citation20,Citation21
Impact of patient-focused perspectives such as QoL
In spite of the advances in immune checkpoint inhibitors for NSCLC treatment, due to unrestrained T-cell activation, immunotherapy can lead to manifestations of toxicity, such as autoimmune breakthrough or immune-related adverse events (irAEs).Citation52 During the past 2 decades until 2010, 32 mAbs have been approved by the FDA for use as drugs, but two of the three drugs that might be the focus of clinical trials have been removed from the market due to the occurrence of severe AEs in human patients.Citation53
Both education and communication among patients, caregivers, and the clinical team are vital for appropriate recognition and management of irAEs.Citation52 For instance, the most common AEs in patients who receive ipilimumab include fatigue, diarrhea, rash, pruritus, and colitis. In addition, irAEs that result from the use of PD-1 inhibitors are similar. In an assessment of patients with NSCLC who were treated with immune checkpoint inhibitors, it is extremely important to recognize that immunotherapy is different from chemotherapy; the irAEs observed with immunotherapy have a completely distinct underlying mechanism compared with the toxicity that is observed with chemotherapy.Citation52
In this context, even though the OS is an important outcome with respect to which treatment a patient should receive, the possible AEs and the symptomatic benefits of therapy must always be considered. Although assessment of QoL is greatly important to patients and clinicians, the evaluation of QoL data is a feature in a minority of trials of patients with NSCLC, and has contributed to the failure of lung cancer research.Citation54
Some tools help researchers to assess QoL. Among them, the Lung Cancer Symptom Scale consists of a lung cancer-specific measure of QoL. This scale is used particularly in clinical trials and assesses six common symptoms associated with lung cancer, such as loss of appetite, fatigue, cough, dyspnea, hemoptysis, and pain.Citation55 This evaluation involves the use of the visual analog scale. Furthermore, other methods include the background demographic questionnaire and the Palliative Performance Scale. The latter, which is adapted from the Karnofsky Performance Rating Scale, rates physical performance and has five functional dimensions: ambulation, activity level and evidence of disease, self-care, oral intake, and level of consciousness. The Karnofsky Performance Rating Scale ranged from 0% (death) to 100% (fully ambulatory and healthy).Citation55
Positive outcomes including improved QoL, improved mood, care that is directed less at an increase in lifespan, and longer survival have been demonstrated from the delivery of palliative care services that are integrated into oncology care among patients with advanced cancers.Citation24 Reinke et al found that, among patients with stage IV lung cancer, the weekly assessment of symptoms in the outpatient setting by a palliative care team improved the QoL and the symptom burden and increased survival, compared with patients in a traditional treatment setting.Citation24
Currently, with attention focused on immune checkpoint inhibitors, several questions about irAEs will likely be resolved with more widespread clinical trials. It is not clear, for instance, if autoimmune disease is an absolute contraindication for this type of therapy. In this context, studies that identify biomarkers and other factors involved in response and resistance to immunotherapy, in addition to trials that assess the combination of immunotherapy and chemotherapy, targeted therapy, or multiple immune modulators, are underway to better define this treatment modality for cancer.Citation52
Conclusion
Considering all the aspects discussed in this manuscript, it is noteworthy that immuno checkpoint inhibitors have established a new era for the treatment of advanced NSCLC. The large set of susceptible patients for this scenario emerges concurrently with the competitive research trials among the main pharmaceutical sponsors and clinical research officers in an attempt to find the best setting for their innovative drugs. Nevertheless, this is a very difficult task because the data change rapidly and sometimes show the limitations and important concerns of the current trial designs. The major sponsors and clinical research officers have launched several of the current trials in the last 3–5 years. This has led to the identification of potential limitations, such as the biomarker selection implementation (eg, in the case of PD-L1 expression as an inclusion criterion, which would restrict patient selection and possible benefits), as well as the image response criteria assessment protocols (eg, CheckMate 063 assessed the ORR using the traditional Response Evaluation Criteria in Solid Tumors version 1.1 criteria, which was not appropriate for immunotherapy).Citation56 Advances in research are occurring more quickly than changes in traditional practices, and perhaps, some protocol amendments are difficult to implement in such multicentric randomized control trials. However, the data regarding the outcomes, toxicity profile control, cost-effective analysis, and patient QoL have emerged in an attempt to determine the best approaches to improve patient care.
Disclosure
RAM has received honoraria from the Pfizer Advisory Board, Zodiac Advisory Board, AstraZeneca, and the National Science Centre (Krakow, Poland), and an educational grant from Pierre Fabre. RAM is an ad hoc consultant at the Ministry of Health, Brasília, Brazil. The other authors have no conflicts of interest related to this manuscript.
References
- TorreLABrayFSiegelRLFerlayJLortet-TieulentJJemalAGlobal cancer statistics, 2012CA Cancer J Clin20156528710825651787
- ThomasAGiacconeGWhy has active immunotherapy not worked in lung cancer?Ann Oncol201526112213222026232492
- La-BeckNMJeanGWHuynhCAlzghariSKLoweDBImmune checkpoint inhibitors: new insights and current place in cancer therapyPharmacotherapy2015351096397626497482
- AdaGThe coming of age of tumour immunotherapyImmunol Cell Biol199977218018510234555
- BorghaeiHPaz-AresLHornLNivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancerN Engl J Med2015373171627163926412456
- ReimanJMKmieciakMManjiliMHKnutsonKLTumor immunoediting and immunosculpting pathways to cancer progressionSemin Cancer Biol200717427528717662614
- SmythMJThiaKYStreetSEMacGregorDGodfreyDITrapaniJAPerforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphomaJ Exp Med2000192575576010974040
- PageDBBourlaABDaniyanATumor immunology and cancer immunotherapy: summary of the 2014 SITC primerJ Immunother Cancer20153125
- SchreiberRDOldLJSmythMJCancer immunoediting: integrating immunity’s roles in cancer suppression and promotionScience201133160241565157021436444
- GiacconeGBazhenovaLANemunaitisJA phase III study of belagenpumatucel-L, an allogeneic tumour cell vaccine, as maintenance therapy for non-small cell lung cancerEur J Cancer2321511623212329
- ButtsCSocinskiMAMitchellPLTecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trialLancet Oncol2014151596824331154
- RibasAReleasing the brakes on cancer immunotherapyN Engl J Med2015373161490149226348216
- AguiarPNJrSantoroILTadokoroHA pooled analysis of nivolumab for the treatment of advanced non-small-cell lung cancer and the role of PD-L1 as a predictive biomarkerImmunotherapy2016891011101927485075
- MadureiraPde MelloRAde VasconcelosAZhangYImmunotherapy for lung cancer: for whom the bell tolls?Tumor Biol201536314111422
- ErnstBAndersonKSImmunotherapy for the treatment of breast cancerCurr Oncol Rep2015172525677118
- ShankaranVIkedaHBruceATIFNγ and lymphocytes prevent primary tumour development and shape tumour immunogenicityNature200141068321107111111323675
- DigheASRichardsEOldLJSchreiberRDEnhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptorsImmunity1994164474567895156
- BurnetMCancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applicationsBr Med J19571502384184713413231
- ThomasLOn immunosurveillance in human cancerYale J Biol Med1982553–43293336758376
- DunnGPBruceATIkedaHOldLJSchreiberRDCancer immunoediting: from immunosurveillance to tumor escapeNat Immunol200231199199812407406
- DunnGPOldLJSchreiberRDThe immunobiology of cancer immunosurveillance and immunoeditingImmunity200421213714815308095
- StaggJJohnstoneRWSmythMJFrom cancer immunosurveillance to cancer immunotherapyImmunol Rev200722018210117979841
- KhongHTRestifoNPNatural selection of tumor variants in the generation of “tumor escape” phenotypesNat Immunol2002311999100512407407
- ReinkeLFFeemsterLCBackhusLMGylys-ColwellIAuDHAssessment and management of symptoms for outpatients newly diagnosed with lung cancerAm J Hosp Palliat Care201633217818325376224
- AntoniaSGettingerSChowLMNivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: interim Phase I resultsJ Clin Oncol201432Suppl 15 Abstract 8023
- StewartTJAbramsSIHow tumours escape mass destructionOncogene200827455894590318836470
- PrendergastGCSmithCThomasSIndoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancerCancer Immunol Immunother201463772173524711084
- VeselyMDKershawMHSchreiberRDSmythMJNatural innate and adaptive immunity to cancerAnnu Rev Immunol20112923527121219185
- PostowMACallahanMKWolchokJDImmune checkpoint blockade in cancer therapyJ Clin Oncol201533171974198225605845
- DominguesDTurnerASilvaMDImmunotherapy and lung cancer: current developments and novel targeted therapiesImmunotherapy20146111221123525496336
- Ostrand-RosenbergSHornLAAlvarezJANovel strategies for inhibiting PD-1 pathway-mediated immune suppression while simultaneously delivering activating signals to tumor-reactive T cellsCancer Immunol Immunother201564101287129325792524
- GhoshRKSharmaASharmaNImmune checkpoint inhibitors in advanced nonsmall cell lung cancerCurr Opin Oncol201527210811725602683
- PatelSPKurzrockRPD-L1 expression as a predictive biomarker in cancer immunotherapyMol Cancer Ther201514484785625695955
- DempkeWCMSellmannLFenchelKEdvardsenKImmunotherapies for NSCLC: are we cutting the gordian helix?Anticancer Res201535115745575726503995
- MayorMYangNStermanDJonesDRAdusumilliPSImmunotherapy for non-small cell lung cancer: current concepts and clinical trialsEur J CardioThoracic Surg201649513241333
- AngYLETanH-LSooRABest practice in the treatment of advanced squamous cell lung cancerTher Adv Respir Dis20159522423525902866
- RizviNAMazièresJPlanchardDActivity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trialLancet Oncol201516325726525704439
- LeDTUramJNWangHPD-1 blockade in tumors with mismatch-repair deficiencyN Engl J Med2015372262509252026028255
- YaqubFNivolumab for squamous-cell non-small-cell lung cancerLancet Oncol2015167e319
- RoundsAKolesarJNivolumab for second-line treatment of metastatic squamous non-small-cell lung cancerAm J Health Syst Pharm201572211851185526490818
- MokTSKLoongHHAre we ready for immune checkpoint inhibitors for advanced non-small-cell lung cancer?Lancet2016387100271488149026712085
- GaronEBRizviNAHuiRPembrolizumab for the treatment of non-small-cell lung cancerN Engl J Med2015372212018202825891174
- SwaikaAHammondWAJosephRWCurrent state of anti-PD-L1 and anti-PD-1 agents in cancer therapyMol Immunol201567241725749122
- HerbstRSBaasPKimD-WPembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trialLancet2016387100271540155026712084
- MonteiroIDCalifanoRMountziosGde MelloRAImmunotherapy with checkpoint inhibitors for lung cancer: novel agents, biomarkers and paradigmsFuture Oncol201612455156426776915
- GaronEBCurrent perspectives in immunotherapy for non-small cell lung cancerSemin Oncol201542Suppl 2S11S1826477470
- LeeJSCollardHRImmunotherapy: the third wave in lung cancer treatmentLancet Respir Med201531292392426679022
- RizviNChaftJBalmanoukianATumor response from durvalumab (MEDI4736) + tremelimumab treatment in patients with advanced non-small cell lung cancer (NSCLC) is observed regardless of PD-L1 statusJ Immunother Cancer20153Suppl 2P193
- SocinskiMAIncorporating immunotherapy into the treatment of non-small cell lung cancer: practical guidance for the clinicSemin Oncol201542Suppl 2S19S2826477471
- JohnsonDBRiothMJHornLImmune checkpoint inhibitors in NSCLCCurr Treat Options Oncol201415465866925096781
- ZatloukalPHeoDSParkKRandomized phase II clinical trial comparing tremelimumab (CP-675,206) with best supportive care (BSC) following first-line platinum-based therapy in patients (pts) with advanced non-small cell lung cancer (NSCLC)J Clin Oncol200927Suppl 15 abstr 8071
- VilladolidJAminAImmune checkpoint inhibitors in clinical practice: update on management of immune-related toxicitiesTransl Lung Cancer Res20154556057526629425
- WangJZouZ-HXiaH-LStrengths and weaknesses of immunotherapy for advanced non-small-cell lung cancer: a meta-analysis of 12 randomized controlled trialsPLoS One201273e3269522403699
- BrownTPilkingtonGBolandAClinical effectiveness of first-line chemoradiation for adult patients with locally advanced non-small cell lung cancer: a systematic reviewHealth Technol Assess2013176199
- O’MahonySNathanSMohajerRSurvival prediction in ambulatory patients with stage III/IV non-small cell lung cancer using the palliative performance scale, ECOG, and lung cancer symptom scaleAm J Hosp Palliat Care201633437438025670717
- de MelloRAPousaIPereiraDNivolumab for advanced squamous cell lung cancer: what are the next steps?Lancet Oncol201516323423525704436
