Abstract
Purpose
Taxane-containing induction chemotherapy (IC) regimens in combination with concurrent chemoradiotherapy (CCRT) have been compared with non-taxane-containing IC combined with CCRT in randomized controlled trials (RCTs) in Chinese patients with advanced nasopharyngeal carcinoma (NPC). This meta-analysis aimed to systematically evaluate their clinical efficacy and safety profiling in this ethnic population.
Methods
The electronic databases, PubMed, Embase, MEDLINE, and Chinese Biomedical Database, were searched for eligible studies. The outcomes included overall response rate (ORR), 1-year survival rate, and different types of adverse events. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of the associations.
Results
A total of 12 RCTs (representing 835 patients) were identified. The pooled analysis showed that taxane-containing regimens had a significant improvement in ORR for nasopharyngeal lesion (OR =4.57, 95% CI =1.14–18.30, P=0.032, z=2.15) but not in cervical lymph nodes (OR =1.23, 95% CI =0.65–2.36, P=0.532, z=0.64) and in 1-year survival rates (OR =1.19, 95% CI =0.10–14.82, P=0.893, z=0.13) compared with non-taxane-containing regimens. Regarding the adverse events and toxicities, grade 3–4 leukopenia and neutropenia were significantly different between the two groups (P<0.001) in favor of the non-taxane-containing regimens, but grade 3–4 vomiting was significantly different between the two groups (P<0.005) in favor of the taxane-containing regimens.
Conclusion
When combined with CCRT, taxane-containing IC regimens may be more efficient for short-term local control in Chinese patients with locally advanced NPC than the non-taxane-containing IC regimens. Moreover, the major toxic effects, which were bone marrow suppression, could be tolerated by majority of patients. More long-term follow-up and high-quality trials of NPC are needed to validate our findings.
Introduction
Nasopharyngeal carcinoma (NPC), a squamous-cell carcinoma, is more prevalent in northern Africa, Alaska, Southeast Asia, and in particular, in the Guangdong area localized at southern part of People’s Republic of China,Citation1 where its incidence is estimated as 80 cases per 100,000, which leads to great threat to the health of affected people.Citation2 Meanwhile, it is noteworthy that the incidence of NPC is higher among Chinese people immigrated to Southeast Asia or North America but is lower among those born in North America than born in southern part of People’s Republic of China.Citation3 Obviously, there is an urgent need to improve treatment outcomes of NPC patients.
NPC is generally considered to be radio- and chemo-sensitive in patient care. Several randomized controlled trials (RCTs) have indicated that use of the radiation therapy in combination with chemotherapy synchronously or sequentially is efficient for NPC patients.Citation5,Citation6 Currently, concomitant use of radiotherapy and chemotherapy (or called concurrent chemoradiotherapy [CCRT]) is generally recognized as the standard of treatment for locally advanced NPC.Citation7 However, clinical observations have shown that ~30%–50% of NPC patients may have metastasis or relapse due to inability to achieve remission, suggesting that current therapy regimen can only control 50%–70% of locally advanced NPC cases successfully.Citation8,Citation9 Furthermore, RCTs have demonstrated that CCRT in combination with adjuvant chemotherapy provides a 31% increase in 3-year overall survival (OS) compared with CCRT alone.Citation10 The 2014 National Comprehensive Cancer Network (NCCN) guidelines recommended that CCRT followed by induction chemotherapy (IC) be acceptable for locally advanced NPC (T1N1-3/T2-4Nx).Citation11 Despite these advances, standard treatment regimens of IC have not been established as the first-line therapy because any of the following IC regimens could be chosen: docetaxel/cisplatin/fluorouracil, cisplatin/epirubicin/paclitaxel, and cisplatin/fluorouracil, the majority of which are taxane-containing regimens.
Taxane is a class of microtubule inhibitors, including paclitaxel and docetaxel (a taxol analog), which works by interfering with cell division. Some evidence has showed that tumor cells would have an increased sensitivity to radiotherapy when patients are treated with docetaxel.Citation12 Furthermore, a literature-based meta-analysis indicated the taxane-containing IC regimen (such as combined use of taxane, cisplatin, and fluorouracil) is superior to that not containing taxane (such as combined use of cisplatin and fluorouracil) with respect to progression-free survival and OS in patients with head and neck cancers recruited in randomized trials,Citation13 suggesting improved clinical outcomes by taxane. However, there were few discussions on taxane-containing IC regimen in NPC treatment, which compared clinical efficacy and safety of taxane-containing IC plus CCRT with those of non-taxane-containing IC plus CCRT in locally advanced NPC.Citation14–Citation25 Here, we aimed to give an overview of all the eligible RCTs for estimated effects of paclitaxel or docetaxel, and to further establish the role of taxane in the standard of IC regimens for locally advanced NPC.
Materials and methods
Study design
To elucidate the inclusion criteria, a protocol was written before literature search. The eligible trials had to meet the following inclusion criteria: the trials should be RCTs, and patients with locally advanced NPC (stage III–IV) should be treated with CCRT in combination with taxane-containing IC or non-taxane-containing IC, without any interference with other treatments. Retrospective studies and prospective studies were used in this meta-analysis.
Literature search
Electronic databases, such as PubMed, Embase, MEDLINE, and Chinese Biomedical Database, were searched for eligible studies. The search terms included the following keywords: ([nasopharyngeal carcinoma OR nasopharyngeal cancer OR cancer of nasopharynx OR NPC OR NPCa OR nasopharyngeal neoplasms] AND [paclitaxel OR docetaxel OR taxanes OR taxane OR aisui OR dopafei OR taxotere OR taxol] AND [RCTs OR randomized controlled trial]). The language of literature was limited to English and Chinese. In addition, we used the PubMed option “Related Articles” in each paper to retrieve potentially relevant ones, and manually searched the references of all the included articles.
Data extraction
Two researchers independently performed data extraction (R Tian and BG Zhang) using a standard extraction form. To resolve discrepancies, group consensus and consulting with a third researcher were used (DY Gu). If the eligibility of the abstract was unclear, the full article was retrieved for clarification. Any problems were solved by discussion.
The deadline for trial inclusion was October 14, 2014.
Statistical analysis
The primary clinical end point was OS, defined as the time from start of randomization until death from any cause. The secondary end points were overall response rate (ORR) and adverse drug reactions.
We used odds ratios (ORs) and 95% confidence intervals (CIs) to evaluate the strength of the associations. Short-term efficacy, 1-year survival rate, and different types of adverse events were compared with ORs and 95% CIs using χ2 test. Both the Cochran’s Q statistic (testing for heterogeneity) and I2 statistic (quantifying the proportion of the total variations due to heterogeneity) were used to estimate heterogeneity across all individual studies included in this meta-analysis.Citation26,Citation27 If a P-value of the Q test was <0.05, indicating a lack of heterogeneity across studies, the summary OR estimate of each study was calculated by the fixed-effects model (the Mantel–Haenszel method);Citation28 otherwise, random-effects model (the DerSimonian and Laird method) was used. STATA Version 10.0 was used for statistical analysis. All reported P-values were two-sided.Citation29
Results
Description of included studies
Clinical characteristics of recruited NPC patients are summarized in . The eligible 12 RCTs included one study in English and eleven studies in Chinese, which have been published in the Journals of Statistic Source.Citation14–Citation25 A total of 835 patients were enrolled. Among those, 412 (49.3%) received the taxane-containing IC regimens, and the rest received non-taxane-containing regimens in addition to CCRT. The sample size ranged from 21 to 76. All patients were diagnosed with advanced NPC (stage III–IV) histologically or pathologically. All studies were case–control studies, with records of complete remission (CR), partial release (PR), 1-year survival rates, and toxicities.
Table 1 Clinical characteristics of patients with nasopharyngeal carcinoma
Short-term efficacy
Clinical efficacy on primary nasopharyngeal lesion and metastatic lesions of cervical lymph nodes was evaluated for the taxane-containing regimen group and non-taxane-containing regimen group of NPC patients at 3 months after IC in combination with CCRT. As shown in , the pooled analysis of the short-term efficacy was performed, in which data on CR and PR of primary nasopharyngeal lesions were assessed in all 12 studies, and metastatic lesions of cervical lymph nodes were evaluated in eight of 12 studies. As shown in , taxane-containing IC regimen had a significantly increased ORR for nasopharyngeal lesion (OR =4.57, 95% CI =1.14–18.30, P=0.032, z=2.15) but not for cervical lymph nodes (OR =1.23, 95% CI =0.65–2.36, P=0.532, z=0.64).
Figure 1 Forest plot of the overall response rate for asopharyngeal lesion.
Abbreviation: CI, confidence interval.
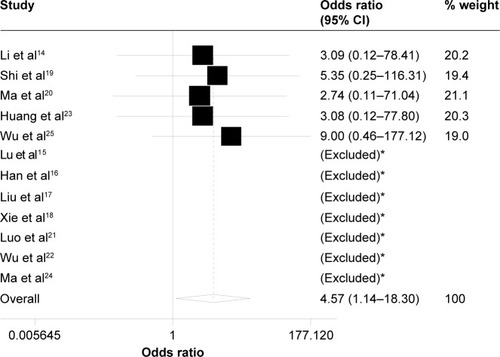
Table 2 The short-term efficacy in two groups of the included studies
Survival profile
Survival analyses showed that 1-year survival rate of taxane-containing IC regimens did not significantly differ from that of non-taxane-containing IC regimens (98.5% versus 96.2%; OR =1.19, 95% CI =0.10–14.82, P=0.893, z=0.13).
Hematological adverse events
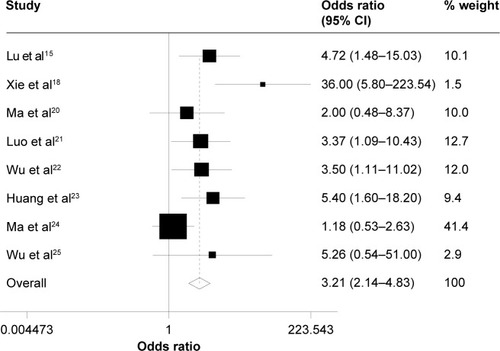
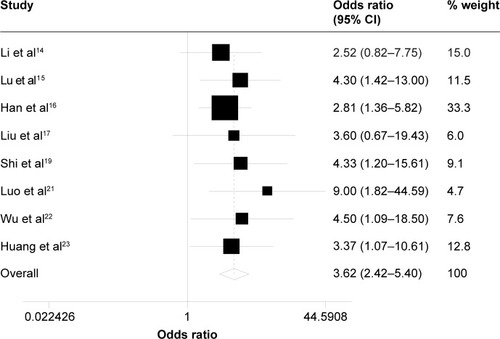
The incidences of adverse events are summarized in . The occurrence rates of grade 3 and higher leukopenia were 46.7% and 22.4%, and those of grade 3–4 neutropenia were 39.1% and 16.1% in testing group and control group, respectively. As shown in and , patients treated with taxane-containing IC regimens in combination with CCRT had a significantly high incidence of grade 3–4 leukopenia (OR =3.21, 95% CI =2.14–4.83, P<0.001, z=5.63) – and neutropenia (OR =3.62, 95% CI =2.42–5.40, P<0.001, z=6.29) compared to those treated with non-taxane-containing IC regimens. Meanwhile, there were no significant differences in the occurrence rate of other hematological adverse events, such as anemia (OR =1.46, 95% CI =0.81–2.63, P=0.214, z=1.24) and thrombocytopenia (OR =1.67, 95% CI =0.99–2.83, P=0.055, z=0.92) between these two groups.
Table 3 The incidences of adverse events of the included studies
Non-hematological adverse events
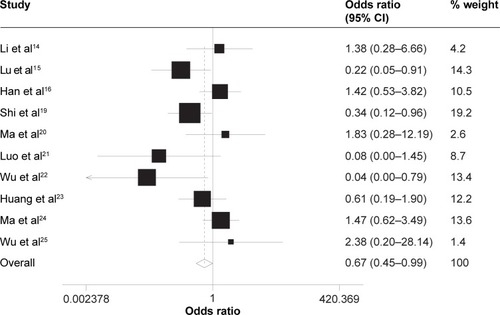
Grade 3–4 vomiting (OR =0.67, 95% CI =0.45–0.99, P=0.043, z=2.03) was observed significantly different between the two groups in favor of the taxane-containing group (). There were similar incidences of other non-hematological toxicities between the two groups of patients receiving CCRT with or without the taxane-containing IC regimen, such as alanine aminotransferase elevation (OR =1.45, 95% CI =0.70–2.98, P=0.315, z=0.78), oral mucositis (OR =1.27, 95% CI =0.89–1.82, P=0.182, z=0.74), dermatitis (OR =0.98, 95% CI =0.49–1.99, P=0.963, z=0.05), and dry mouth (OR =0.96, 95% CI =0.33–2.81, P=0.941, z=0.09).
Risk of bias assessment
Data on the incidence of vomiting indicated a lack of heterogeneity across studies (P=0.013); thus, the random-effects model was used. The fixed-effects model was used in the incidence of other adverse events, short-term efficacy, and 1-year OS.
Discussion
This study is the first RCT-based meta-analysis that evaluated clinical efficacy and safety of taxane-containing versus non-taxane-containing IC regimens in combination with CCRT regimen for locally advanced NPC in Chinese people, revealing remarkably improved ORR for nasopharyngeal lesion. In addition, the two IC regimens were well tolerated by all patients, although grade 3–4 vomiting was observed to be significantly different between the two groups in favor of the taxane-containing regimen, and grade 3–4 leukopenia and neutropenia were significantly different in favor of the non-taxane-containing regimen.
A few studies have focused on the taxane-containing IC regimen in combination with CCRT for NPC. In 2009, Hui et alCitation30 reported a Phase II trial of 60 patients with NPC (stage III–IVB), who were randomly assigned to receive CCRT alone or IC (docetaxel and cisplatin) subsequently in combination with CCRT, and observed a significantly improved 3-year OS for the latter with a manageable toxicity profile compared with CCRT alone. Bossi et alCitation31 reported a single-center study evaluating clinical efficacy and safety of IC regimen (docetaxel, cisplatin, and 5-fluorouracil) in combination with CCRT for Epstein–Barr virus-related locally advanced NPC, and revealed tolerated toxicity of the IC regimen of interest. These studies suggested that docetaxel is the major cancer drug in the taxane-containing IC regimens for advanced NPC, which is more efficient and well tolerated in patient care, consistent with the findings of our meta-analysis.
Some argument concerns that different IC and CCRT regimens and drugs utilized in each research might influence the comparison of the functions of taxane. In our meta-analysis, the conclusion of each independent research is obtained when expelling other factors which can interfere with the observation. Each independent research is focused on addressing the magnitude of the benefit and safety of taxane-containing regimen in advanced NPC treatment in Chinese population, which has been expelling other influencing factors.
In addition, of the hematologically adverse events, the incidence of grade 3–4 leukopenia and neutropenia was remarkably different between the two groups in favor of non-taxane-containing regimen, which is consistent with previous studies.Citation32,Citation33 But it was reported that neutropenia caused by taxane combined with cisplatin IC regimen would rebound quickly after using granulocyte colony-stimulating factor processing, while neutropenia caused by cisplatin combined with fluorouracil regimen recovered slower, leading to extended or interrupted treatment, although the rate of hematological toxicities was lower.Citation34 A number of clinical research studies have demonstrated that much higher proportion of NPC patients could complete all the chemotherapy,Citation15 or much more courses of chemotherapy could be completedCitation18,Citation21,Citation22 when patients received taxane-containing IC regimens than non-taxane-containing IC regimens, indicating that a higher completion rate of the taxane-containing IC regimens may be due to good tolerance.
Our meta-analysis indicated that the taxane-containing regimen has a significantly improved ORR for nasopharyngeal lesion but that its other short-term efficacy and survival profiles are similar to those of non-taxane-containing IC regimens. This is mainly because of the limitations to this meta-analysis. First of all, these RCTs included different taxane-containing IC regimens using two types of different taxanes: docetaxel or paclitaxel. However, the benefits and side effects of taxane-containing regimen do not seem to vary by the taxane. Second, two different types of CCRT regimens are used during radiation in these trials: taxane and cisplatin. Third, since the IC is used for a short period of time in NPC, the included trials have a short follow-up with no data on the relapse. Thus, we could only compare the short-term efficacy and 1-year survival profile between the two groups for NPC treatment. Last but most importantly, all NPC patients included in this meta-analysis are Chinese people, and the sample size is not large enough. The conclusions we have made cannot be extrapolated to NPC patients of other ethnic backgrounds.
Conclusion
Limited RCTs have showed that taxane belongs to a class of clinically efficient antitumor drugs for locally advanced NPC, and that the taxane-containing IC regimen is superior to the non-taxane-containing regimen as assessed by a higher ORR in the former for primary nasopharyngeal lesions. In the future, more multicenter, large-scaled, high-quality RCTs are required to replicate and even confirm these findings in Chinese or other ethnic populations.
Acknowledgments
The authors thank Professor Hong-Guang Xie, General Clinical Research Center, Nanjing First Hospital, Nanjing Medical University, People’s Republic of China, for his critical reading of the manuscript and language editing.
Disclosure
The authors report no conflicts of interest in this work.
References
- WeiWIShamJSNasopharyngeal carcinomaLancet200536594762041205415950718
- ChanATTeoPMJohnsonPJNasopharyngeal carcinomaAnn Oncol20021371007101512176778
- DicksonRIFloresADNasopharyngeal carcinoma: an evaluation of 134 patients treated between 1971–1980Laryngoscope19859532762833974378
- BuellPThe effect of migration on the risk of nasopharyngeal cancer among ChineseCancer Res1974345118911914842361
- ChanATLeungSFNganRKOverall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinomaJ Natl Cancer Inst200597753653915812080
- WeeJTanEHTaiBCRandomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on cancer/International Union against cancer stage III and IV nasopharyngeal cancer of the endemic varietyJ Clin Oncol200523276730673816170180
- ChanATHuiEPLeungSFNasopharyngeal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-upAnn Oncol200718suppl 2ii67ii6817491054
- ChuaDTShamJSWeiWIHoWKAuGKThe predictive value of the 1997 American Joint Committee on cancer stage classification in determining failure patterns in nasopharyngeal carcinomaCancer200192112845285511753958
- ChuaDTShamJSWeiWIHoWKAuGChoyDControl of regional metastasis after induction chemotherapy and radiotherapy for nasopharyngeal carcinomaHead Neck200224435036011933177
- Al-SarrafMLeBlancMGiriPGChemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: phase III randomized Intergroup study 0099J Clin Oncol1998164131013179552031
- National Comprehensive Cancer Network. NCCN Guidelines® Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed July 9, 2015
- NabellLSpencerSDocetaxel with concurrent radiotherapy in head and neck cancerSemin Oncol2003306 suppl 18899314727247
- BlanchardPBourhisJLacasBMeta-Analysis of Chemotherapy in Head and Neck Cancer, Induction Project, Collaborative GroupTaxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the meta-analysis of chemotherapy in head and neck cancer groupJ Clin Oncol201331232854286023835714
- LiXPBaiGPWeiNThe short-term effect of TPF, PF induction chemotherapy followed by chemoradiotherapy for local advanced nasopharyngeal carcinomaJ Mod Oncol20122020312033
- LuXGuoXHongMHChenQYZengQXiangYQComparison of the short-term efficacy of two inductive chemotherapy regimens for locally advanced nasopharyngeal caricinoma: docetaxal plus carboplatin versus 5-fluorouracil plus carboplatinChin J Cancer20102914014420109340
- HanSHYuLZhangZZhangPJSongHPGuoCYEvaluation of induction chemotherapy with vinorelbine plus cisplatin (NP) or docetaxel plus cisplatin (TP) combined with concurrent chemoradiotherapy for patients with locally advanced nasopharyngeal carcinomaZhonghua Zhong Liu Za Zhi201335862362624314223
- LiuGCHuXFHuangGSA clinical research on the TPF or PF neo-adjuvant chemotherapy combined with concurrent chemoradiotherapy in the treatment of locally advanced nasopharyngeal carcinomaAnti-Tumor Pharm20133204212
- XieFYQiSNHuWHZouGRPengMLiJSComparison of efficacy of docetaxel combined cisplatin (TP regimen) and cisplatin combined 5-fluorouracil (PF regimen) on locally advanced nasopharyngeal carcinomaAi Zheng200726888088417697552
- ShiMHChengJFWangLComparison of efficacy of induction chemotherapy between docetaxel combined cisplatin (TP) and cisplatin combined 5-fluorouracil (TP) for locally advanced nasopharyngeal carcinomaModern Oncology201422537539
- MaJJZhangYBZhaoMHShort-term treatment effect comparison of TPF, PF induction chemotherapy followed by chemoradiotherapy for local advanced nasopharyngeal carcinomaAnhui Med Pharm J20111512911293
- LuoJHLinYZhouJClinical study of inductive chemotherapy with docetaxel plus nedaplatin followed by concurrent nedaplatin with radiotherapy for advanced nasopharyngeal carcinomaTumorsci201131523537
- WuFWangRSWeiBZhangYLiuWQClinical efficacy of inductive chemotherapy with docetaxel plus nedaplatin followed by concurrent nedaplatin with radiotherapy for locally advanced nasopharyngeal carcinomaHerald Med201332459463
- HuangSNWangRSLiangFFDuQHInductive chemotherapy followed by chemoradiotherapy for locally advanced nasopharyngeal carcinomaChin J Cancer Prev Treat201320614617
- MaHMYuanXHuangYLLinYFLiuCXPaclitaxel and cisplatin, 5-fluorouracil (TPF) programme neoadjuvant chemotherapy for advanced nasopharyngeal cancerMod Oncol20091712331235
- WuJTPengJYWuCBDingJTThe efficacy of induction chemotherapy with TPF scheme for locally advanced nasopharyngeal carcinomaPract J Cancer201429143145
- CochranWGThe comparison of percentages in matched samplesBiometrika1950373–425626614801052
- HigginsJPThompsonSGDeeksJJAltmanDGMeasuring inconsistency in meta-analysesBMJ2003327741455756012958120
- MantelNHaenszelWStatistical aspects of the analysis of data from retrospective studies of diseaseJ Natl Cancer Inst195922471974813655060
- DerSimonianRLairdNMeta-analysis in clinical trialsControl Clin Trials1986731771883802833
- HuiEPMaBBLeungSFRandomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinomaJ Clin Oncol200927224224919064973
- BossiPOrlandiEBergaminiCDocetaxel, cisplatin and 5-fluorouracil-based induction chemotherapy followed by intensity-modulated radiotherapy concurrent with cisplatin in locally advanced EBV-related nasopharyngeal cancerAnn Oncol201122112495250021398385
- TsukudaMMikamiYTanigakiYPhase I trial of combined chemotherapy with docetaxel, cisplatin, and 5-fluorouracil for patients with locally advanced squamous cell carcinoma of the head and neckInt J Clin Oncol20049316116615221599
- GenetDCupissolDCalaisGDocetaxel plus 5-fluorouracil in locally recurrent and/or metastatic squamous cell carcinoma of the head and neck: a phase II multicenter studyAm J Clin Oncol200427547247615596913
- KwongDLShamJSAuGKConcurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: a factorial studyJ Clin Oncol200422132643265315226332