Abstract
Purpose
Interleukin-6 (IL-6) plays an important role in human colorectal cancer (CRC) development. However, the exact clinical and prognostic significance of IL-6 in CRC is still unclear. Here, we conducted this meta-analysis to explore this issue in detail.
Methods
A meta-analysis was performed to clarify the association between serum IL-6 expression and clinical outcomes in articles published up to June 2015. Weighted mean difference (WMD) and its corresponding 95% confidence interval (CI) were used to assess the association between serum IL-6 expression and the clinicopathological characteristics of CRC. Hazard ratio (HR) with 95% CI was used to quantify the predictive value of IL-6 on CRC prognosis.
Results
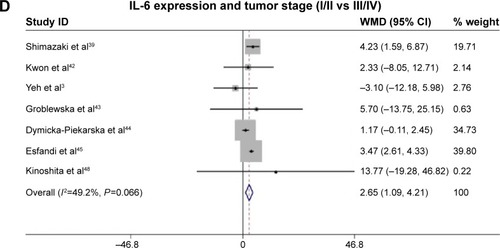
Fourteen studies comprising 1,245 patients were included. Analysis of these data showed that serum IL-6 expression was highly correlated with poor 5-year overall survival (OS) rate (HR =0.43, 95% CI: 0.31–0.59, P=0.755). Simultaneously, we also found that serum IL-6 expression was associated with certain clinical parameters of CRC, such as tumor invasion (T category: T0–T2, T3–T4) (WMD =3.15, 95% CI: 1.92–4.39, P=0.816), distant metastasis (M category: M0, M1) (WMD =4.69, 95% CI: 3.33–6.06, P=0.377), and tumor stage (I–II, III–IV) (WMD =2.65, 95% CI: 1.09–4.21, P=0.066).
Conclusion
A high serum IL-6 expression is associated with adverse OS in CRC. The IL-6 expression can be an important supplement in establishing prognostic score for clinical decision.
Introduction
Colorectal cancer (CRC) is a worldwide disease which is the third most common cause of cancer in both women and men and is also the third most common cause of cancer death after lung and prostate cancer in men and lung and breast cancer in women.Citation1 Inflammation plays an important role in CRC development, including tumor initiation, progression, and metastasis. Numerous inflammatory cytokines have been regarded as potential diagnostic and prognostic markers in CRC. Among these, interleukin-6 (IL-6) seems to be of central importance in human CRC development. IL-6, a 184-amino acid protein with a molecular weight of approximately 20.3 kDa, is a pleiotropic cytokine produced by multiple cells, including monocytes, macrophages, and others cells such as fibroblasts, keratinocytes, endothelial cells, lymphocytes, and tumor cells.Citation2 It plays a crucial role in regulating the inflammation and immune responses.Citation3 Lahm et al first found that IL-6 had a growth-promoting effect on CRC cell lines in vitro in 1992.Citation4 Chung and ChangCitation5 revealed that levels of IL-6 were elevated in the serum and tumor tissues of CRC patients. Moreover, IL-6 expression level is gradually elevated during the progression from colorectal adenoma to carcinoma.Citation6
Nowadays, we know that IL-6 signaling has two significant pathways. IL-6 can bind to its receptor (IL-6Rα) on the cell surface in classic signaling pathway, or binds to soluble IL-6 receptor (sIL-6R) in trans-signaling pathway, and then form a hexameric signaling complex including core-ceptor gp130 (glycoprotein 130, IL-6Rβ) homodimer and two IL-6–IL-6R heterodimers, thus activating the Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) pathway, which regulates tumor proliferation, prevents apoptosis and induces tumor angiogenesis.Citation7–Citation9 Many researchers have approved that IL-6-JAK-STAT3 signaling plays an important role in multiple tumor models including breast, lung, colon, ovarian, and prostate cancer.Citation10 IL-6Rα is only expressed on a limited number of cell types, such as hepatocytes, megakaryocytes and monocytes, macrophages, B-cells, and T-cells, while sIL-6R exists throughout the body. It is suggested that proinflammatory effects of IL-6 are mainly attributed to trans-signaling pathway, while the classic signaling pathway contributes to anti-inflammatory effects.Citation11
In consideration of the important role of IL-6 in tumor development, many researchers were involved in doing related studies, including correlating IL-6 expression with risk, clinicopathological features, and prognosis of CRC. The associated data seemed inconsistent. Several previous meta-analyses which investigated the association between serum IL-6 expressions with CRC risk showed no signifi-cant correlation.Citation12–Citation15 However, the clinical significance and accurate prognostic value of IL-6 in CRC have not been fully assessed. Therefore, we conducted the first meta-analysis aiming to evaluate the value of serum IL-6 as a prognostic marker for CRC and to research the relationship between serum IL-6 and clinical stage of CRC.
Materials and methods
Publication search
This meta-analysis was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines.Citation16 We searched literature from electronic databases PubMed, MEDLINE, and ISI Web of Science up to June 2015. The search terms included “(interleukin 6 or IL-6) and (colorectal or colon or rectal) and (carcinoma or tumor or cancer or neoplasm)”. The reference lists and supplemental materials associated with the studies and review articles were examined manually to further identify any additional relevant publications.
Selection criteria
The studies aiming to explore the association between serum IL-6 expression and CRC were included. The inclusion criteria were as follows: 1) articles evaluating the relationship between preoperative serum IL-6 expression and parameters such as clinicopathological features including Tumor Node Metastasis classification and survival outcome of CRC and 2) full text, original research articles published in English.
Articles were excluded from the analyses based on the following criteria: 1) they were letters to the editor, reviews, comments, duplicated studies, and articles published in books; 2) papers were published in non-English language; 3) the articles focused on the tissue IL-6 expression; 4) the patients included in the studies underwent preoperative chemotherapy (neoadjuvant chemotherapy); 5) insufficient data was extracted from the articles or the full text could not be found.
Data extraction
All data were extracted independently by two investigators. Any further uncertainties were addressed by joint inspection of the papers and discussion. The following data were obtained from each article: the first author; publication year; country; number of patients; method of IL-6 detection; serum IL-6 expression of different T category, N category, distant metastasis (liver metastasis), and tumor stage (I–II, III–IV); the cut-off value of IL-6; and most importantly, the 5-year overall survival (OS) and the 3-year disease-free survival (DFS) rate. The quality of studies was evaluated according to the Newcastle–Ottawa scale.Citation17
The T, N, M category was determined according to the American Joint Committee on Cancer guidelines. Because the cut-off value for IL-6 expression varied among studies, we defined IL-6-high expression values with respect to the original articles. To avoid bias from some studies that had very long-term follow-up data, OS was standardized to include 5 years of follow-up, while DFS was standardized to include 3 years of follow-up in the included studies. If included articles only provided survival data in a Kaplan–Meier curve, the software GetDataGraph Digitizer 2.24 (http://getdata-graph-digitizer.com/) was used to extract the data.
Data analysis
The guidelines proposed by the Meta-Analysis of Observational Studies in Epidemiology group were applied in the statistical analyses. Because most of the including studies compared serum IL-6 expression quantity in different T, N, M category with its stage, weighted mean difference (WMD) with 95% confidence interval (CI) was used to assess the association between IL-6 expression level and clinicopatho-logical characteristics of CRC. Hazard ratios (HR) and their 95% CIs were used to quantify the predictive ability of IL-6 expression level on CRC prognosis. WMD with 95% CI and HR with 95% CI were calculated using STATA 12.0 (Stata Corp LP, College Station, TX, USA). The heterogeneity among the studies was measured using the Q-test and I2 test. Both fixed- and random-effects models could be used in the absence of heterogeneity, but random-effects models were more appropriate when heterogeneity was present. The publication bias was assessed by Begg rank correlation method (software Stata 12.0). All P-values were for a two-sided test and P<0.05 was considered statistically significant.
Results
Search results
The detailed steps of article identification and inclusion and exclusion criteria are described in . In the initial search, 2,052 published articles were identified according to the search strategies. After screening the titles and abstracts, 1,383 articles were excluded because they were beyond the scope of the study (eg, pathological features, OS, or DFS). Among the rest of included articles, 655 studies were excluded because their focus was on IL-6 mRNA, gene polymorphism, signaling pathway, review, experimental animal models, serum IL-6 level after chemotherapy, and non-English articles. Finally, using explicit inclusion and exclusion criteria mentioned above, a total of 14 eligible studies were included in the final meta-analysis.
Characteristics of eligible studies
The details of the 14 eligible studies are listed in . Of these 14 publications, 11 studies assessed the relationships between serum IL-6 expression and CRC clinicopathologic features, while 7 studies evaluated the association of IL-6 expression and CRC prognosis (OS and DFS). Approximately 1,245 participants were enrolled with a median sample size of 89 (range from 24 to 208) per study. Among them, 7 were from East Asia, including Japan (3), China (2), Korea (2), and 1 was from Middle East Iran; 5 were from Europe, including Poland (2), Italy (1), Finland (1), and Greece (1); 1 was from Egypt, Africa. We did not obtain any studies from North and South America. Enzyme-linked immunosorbent assay (ELISA) method was adopted in all the included studies except one study where Bio-Plex premanufactured 27-Plex Cytokine Panel (Bio-Rad, Hercules, CA, USA) was used, which shares the same mechanism with ELISA. Meanwhile, the quality of the 14 included studies was considered accessible according to the Newcastle–Ottawa scale.Citation18
Table 1 Main characteristics of the eligible results
Correlation of serum IL-6 expression with clinicopathological parameters
Serum IL-6 expression was associated with certain clinical parameters of CRC such as tumor category (T category: T0–2, T3–4) (WMD =3.15, 95% CI: 1.92–4.39, P=0.816, and I2=0.0% fixed effect), distant metastasis (M category: M0, M1) (WMD =4.69, 95% CI: 3.33–6.06, P=0.377, and I2=6.6% fixed effect), and tumor stage (I–II, III–IV) (WMD =2.65, 95% CI: 1.09–4.21, P=0.066, and I2=49.2% random effect) ().
Figure 2 The forest plot of weighted mean difference in the meta-analysis.
Abbreviations: IL-6, interleukin-6; CI, confidence interval; TIS, carcinoma in situ; WMD, weighted mean difference.
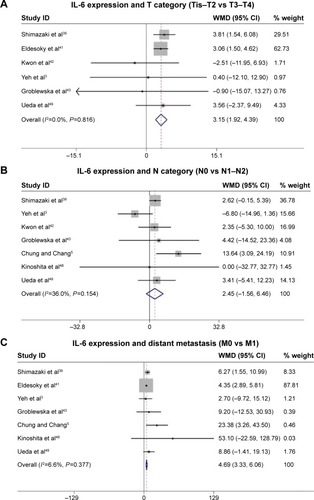
However, there was no statistically significant association between IL-6 expression and lymph node metastasis (N category: N0, N1–2) (WMD =2.45, 95% CI: -1.56 to 6.46, P=0.154, and I2=36.0% random effect) ().
Impact of serum IL-6 expression on 5-year OS and 3-year DFS rates
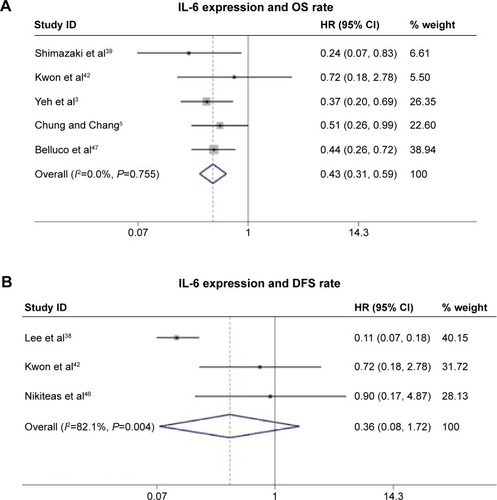
Five studies (408 patients) investigated the association between serum IL-6 expression and OS. The presence of IL-6 expression was highly correlated with poor 5-year OS rate (, HR =0.43, 95% CI: 0.31–0.59, P=0.755, and I2=0.0% fixed effect). This indicated that IL-6 was an independent prognostic factor in CRC. In addition, three studies investigated the association between IL-6 expression and DFS, but no statistically significant association was found with obvious heterogeneity (, HR =0.36, 95% CI: 0.08–1.72, P=0.004, and I2=82.1% random effect).
Publication bias
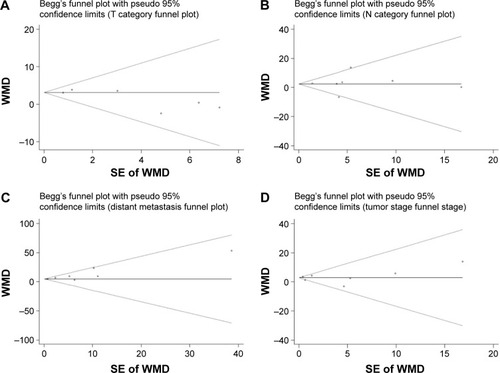
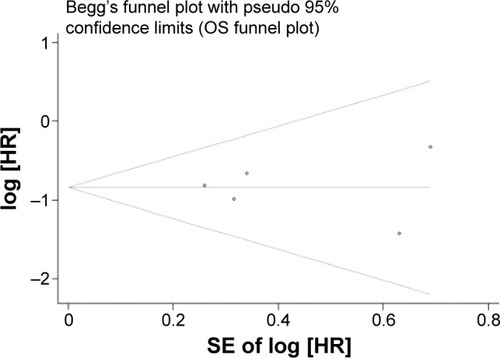
No evidence of potential publication bias for the studies included in our meta-analysis was observed when assessed with Begg’s tests ( and ).
Discussion
In our search strategy, we excluded the studies that focus on serum levels of IL-6 after surgical operation or chemotherapy which may cause deviation. Surgery could increase the body’s stress level, which triggers proinflammatory response, while chemotherapy could decrease the level of IL-6.Citation19
The results from the present meta-analysis showed a poor prognostic outcome in patients expressing high levels of serum IL-6. The results also indicated that IL-6 expression was signifi-cantly associated with tumor local invasion, distant metastasis, and tumor stage. Owing to lack of enough data to perform statistical analysis and the presence of significant heterogeneity (I2=82.1% random effect) from a 3-year DFS rate, we could not reach a specific conclusion approximately 3-year DFS.
In this meta-analysis, IL-6 was significantly associated with the 5-year OS of CRC patients, indicating that IL-6 could promote tumor proliferation. Because of its low molecular weight, IL-6 can freely diffuse through intercellular junction and appear in the tumor microenvironment rapidly, which is the basis for IL-6 to function. IL-6 can promote cancer cell entry into the cell cycle through activating STAT3 which increases the expression of cyclin D1, D2, B1, and c-Myc, and decrease the expression of the cyclin-dependent kinase (Cdk) inhibitor p21.Citation20–Citation23 IL-6 can also increase telomerase activity, thus preventing cellular senescence.Citation24 Besides, IL-6 can promote noncancer cells conversion into cancer stem cells, which possess properties of self-renewal and repopulation potential.Citation25 Furthermore, IL-6 can regulate tumor cell proliferation by interacting with growth factor signaling, including epidermal growth factor family members and hepatocyte growth factor.Citation23 IL-6 also provides a key regulatory signal in the T-cell differentiation pathway, downregulating T-lymphocytic function and impairing adaptive immune response, thus allowing tumor cells to escape immune surveillance.Citation8,Citation26 All of these discoveries illustrated the protumor function of IL-6 via promoting tumor cell growth inordinately and inhibiting apoptosis.
IL-6 has also been found to be significantly associated with tumor local invasion and distant metastasis based on the data from this meta-analysis. IL-6 can induce matrix metalloproteinase (MMP)-2, MMP-7, and MMP-9, which play a role in extracellular matrix degradation and tumor invasion, via activation of the IL-6-JAK-STAT3 pathway. In addition, IL-6 can lead to epithelial–mesenchymal transition, which facilitates cancer cells invasion of other tissues and blood vessels, thus causing distant metastases.Citation27–Citation31 Besides, IL-6 also promotes local invasion and forms distant secondary tumor implants through its proangiogenic function mainly via IL-6-JAK-STAT3 pathway, thus leading to hypoxia inducible factor-1-mediated VEGF-A transcription, as well as endothelial cell proliferation and migration.Citation32–Citation35
Nowadays, the pivotal role of IL-6/STAT3 signaling in CRC development has attracted more and more attention. Inhibiting IL-6 in tumor cells is likely to prevent tumor invasion and metastasis. Several therapeutics inhibiting this pathway have been developed and have shown a promising future for the treatment of human disease during recent years. These include anti-IL-6 agents like siltuximab or anti-IL-6R antibodies like tocilizumab, soluble gp130Fc, and selective small molecule JAK inhibitors like CEP-33779.Citation36,Citation37 Now, tocilizumab has been approved by the US Food and Drug Administration for clinical use. We hope that the preclinical data will reach their promise and become a potential therapy for the cancer patients refractory to conventional chemotherapy drugs.
Some limitations should be considered in this meta-analysis. First, the cut-off value was different between the included studies, which could cause heterogeneity among the studies. Therefore, more studies with the same cut-off values should be recruited and combined for further analysis. Second, because of the lack of data relating the IL-6 expression and DFS, which is also an important aspect reflecting the prognosis, we could not conclude an accurate result now. Third, the lack of concrete data made us unable to further explore the association of CRC with more detailed factors like age via subgroup analysis.
Conclusion
To our knowledge, this meta-analysis is the first study to systematically and quantitatively evaluate the association between serum IL-6 and clinicopathological features and prognostic factors in CRC. Our results showed that serum IL-6 expression has a significant negative correlation with the 5-year OS rates in CRC patients. Besides, serum IL-6 expression was also related to tumor local invasion and distant metastasis. Thus, elevated serum IL-6 expression indicated a poor prognostic significance for CRC patients, and IL-6 expression could be an important supplement in establishing prognostic score for clinical decision. We hope there will be more studies to further evaluate the application of IL-6 as a biomarker for CRC prognosis and to assess whether IL-6 could change in the process of CRC chemotherapy or neoadjuvant therapy.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
- SiegelRLMillerKDJemalACancer statistics, 2015CA Cancer J Clin201565152925559415
- KishimotoTThe biology of interleukin-6Blood19897411102473791
- YehKYLiYYHsiehLLAnalysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancerJpn J Clin Oncol201040658058720194250
- LahmHPetral-MalecDYilmaz-CeyhanAGrowth stimulation of a human colorectal carcinoma cell line by interleukin-1 and -6 and antagonistic effects of transforming growth factor beta 1Eur J Cancer199228a11189418991389533
- ChungYCChangYFSerum interleukin-6 levels reflect the disease status of colorectal cancerJ Surg Oncol200383422222612884234
- BrozekWBisesGGirschTCrossHSKaiserHEPeterlikMRole of interleukin-6 in modulation of human colon carcinoma cell growthAnticancer Res2004245D35983599
- HodgeDRHurtEMFarrarWLThe role of IL-6 and STAT3 in inflammation and cancerEur J Cancer200541162502251216199153
- NauglerWEKarinMThe wolf in sheep’s clothing: the role of interleukin-6 in immunity, inflammation and cancerTrends Mol Med200814310911918261959
- YuHLeeHHerrmannABuettnerRJoveRRevisiting STAT3 signalling in cancer: new and unexpected biological functionsNat Rev Cancer2014141173674625342631
- YaoXHuangJZhongHTargeting interleukin-6 in inflammatory autoimmune diseases and cancersPharmacol Ther2014141212513924076269
- SchellerJChalarisASchmidt-ArrasDRose-JohnSThe pro- and anti-inflammatory properties of the cytokine interleukin-6Biochim Biophys Acta20111813587888821296109
- HeikkilaKHarrisRLoweGAssociations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysisCancer Causes Control2009201152618704713
- JoshiRKKimWJLeeSAAssociation between obesity-related adi-pokines and colorectal cancer: a case-control study and meta-analysisWorld J Gastroenterol201420247941794924976730
- JoshiRKLeeSAObesity related adipokines and colorectal cancer: a review and meta-analysisAsian Pac J Cancer Prev201415139740524528064
- ZhouBShuBYangJLiuJXiTXingYC-reactive protein, interleukin-6 and the risk of colorectal cancer: a meta-analysisCancer Causes Control201425101397140525053407
- LiberatiAAltmanDGTetzlaffJThe PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaborationPLoS Med200967e100010019621070
- StangACritical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analysesEur J Epidemiol201025960360520652370
- WellsGASheaBO’ConnellDThe Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspAccessed July 29, 2015
- GaiollaRDDominguesMANiero-MeloLde OliveiraDESerum levels of interleukins 6, 10, and 13 before and after treatment of classic Hodgkin lymphomaArch Pathol Lab Med2011135448348921466366
- BeckerCFantiniMCSchrammCTGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signalingImmunity200421449150115485627
- GrivennikovSKarinETerzicJIL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancerCancer Cell200915210311319185845
- BollrathJPhesseTJvon BurstinVAgp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesisCancer Cell20091529110219185844
- TaniguchiKKarinMIL-6 and related cytokines as the critical lynchpins between inflammation and cancerSemin Immunol2014261547424552665
- YamagiwaYMengFPatelTInterleukin-6 decreases senescence and increases telomerase activity in malignant human cholangiocytesLife Sci200678212494250216336976
- KimSYKangJWSongXRole of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cellsCell Signal201325496196923333246
- GuthrieGJRoxburghCSHorganPGMcMillanDCDoes interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer?Cancer Treat Rev2013391899622858249
- NietoMAEpithelial plasticity: a common theme in embryonic and cancer cellsScience20133426159123485024202173
- De CraeneBBerxGRegulatory networks defining EMT during cancer initiation and progressionNat Rev Cancer20131329711023344542
- TsaiJHYangJEpithelial-mesenchymal plasticity in carcinoma metastasisGenes Dev201327202192220624142872
- TamWLWeinbergRAThe epigenetics of epithelial-mesenchymal plasticity in cancerNat Med201319111438144924202396
- CreightonCJGibbonsDLKurieJMThe role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspectiveCancer Manag Res2013518719523986650
- MiddletonKJonesJLwinZCowardJIInterleukin-6: an angiogenic target in solid tumoursCrit Rev Oncol Hematol201489112913924029605
- XuQBriggsJParkSTargeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathwaysOncogene200524365552556016007214
- JungJELeeHGChoIHSTAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cellsFASEB J200519101296129815919761
- HsuCPChungYCInfluence of interleukin-6 on the invasiveness of human colorectal carcinomaAnticancer Res2006266b4607461417201185
- JonesSASchellerJRose-JohnSTherapeutic strategies for the clinical blockade of IL-6/gp130 signalingJ Clin Invest201112193375338321881215
- WaldnerMJFoerschSNeurathMFInterleukin-6 – a key regulator of colorectal cancer developmentInt J Biol Sci2012891248125323136553
- LeeWSBaekJHYouDHNamMJPrognostic value of circulating cytokines for stage III colon cancerJ Surg Res20131821495423010514
- ShimazakiJGotoYNishidaKIn patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progressionOncology201384635636123689116
- KantolaTKlintrupKVayrynenJPStage-dependent alterations of the serum cytokine pattern in colorectal carcinomaBr J Cancer2012107101729173623059742
- EldesokyAShoumaAMosaadYElhawaryAClinical relevance of serum vascular endothelial growth factor and interleukin-6 in patients with colorectal cancerSaudi J Gastroenterol201117317017321546718
- KwonKAKimSHOhSYClinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancerBMC Cancer20101020320465852
- GroblewskaMMroczkoBWereszczynska-SiemiatkowskaUSerum interleukin 6 (IL-6) and C-reactive protein (CRP) levels in colorectal adenoma and cancer patientsClin Chem Lab Med200846101423142818844497
- Dymicka-PiekarskaVMatowicka-KarnaJGrykoMKemona-ChetnikIKemonaHRelationship between soluble P-selectin and inflammatory factors (interleukin-6 and C-reactive protein) in colorectal cancerThromb Res2007120458559017169411
- EsfandiFGhobadlooSMBasatiGInterleukin-6 level in patients with colorectal cancerCancer Lett20062441767816442710
- NikiteasNITzanakisNGazouliMSerum IL-6, TNF alpha and CRP levels in Greek colorectal cancer patients: prognostic implicationsWorld J Gastroenterol200511111639164315786541
- BellucoCNittiDFrantzMInterleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancerAnn Surg Oncol20007213313810761792
- KinoshitaTItoHMikiCSerum interleukin-6 level reflects the tumor proliferative activity in patients with colorectal carcinomaCancer199985122526253110375098
- UedaTShimadaEUrakawaTSerum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasisJ Gastroenterol19942944234297951851