Abstract
Intrahepatic cholangiocarcinoma (ICC) is the second most common malignancy arising from the liver. ICC makes up about 10% of all cholangiocarcinomas. It arises from the peripheral bile ducts within the liver parenchyma, proximal to the secondary biliary radicals. Histologically, the majority of ICCs are adenocarcinomas. Only a minority of patients (15%) present with resectable disease, with a median survival of less than 3 years. Multidisciplinary management of ICC is complicated by large differences in disease course for individual patients both across and within tumor stages. Risk models and nomograms have been developed to more accurately predict survival of individual patients based on clinical parameters. Predictive risk factors are necessary to improve patient selection for systemic treatments. Molecular differences between tumors, such as in the epidermal growth factor receptor status, are promising, but their clinical applicability should be validated. For patients with locally advanced disease, several treatment strategies are being evaluated. Both hepatic arterial infusion chemotherapy with floxuridine and yttrium-90 embolization aim to downstage locally advanced ICC. Selected patients have resectable disease after downstaging, and other patients might benefit because of postponing widespread dissemination and biliary obstruction.
Incidence and risk factors
The incidence of intrahepatic cholangiocarcinoma (ICC) in the Western world is approximately one to two per 100,000.Citation1–Citation3 ICC is the second most common malignancy arising from the liver, accounting for 3% of all cases of gastrointestinal cancer.Citation4,Citation5 ICC makes up about 10% of all cholangiocarcinomas. It arises in peripheral bile ducts within the liver parenchyma, proximal to the secondary biliary radicals ().Citation6 It should be distinguished from perihilar cholangiocarcinoma arising near the biliary confluence and distal cholangiocarcinoma arising near the head of the pancreas. Only a minority (15%) of ICC patients present with resectable disease at the time of diagnosis. Complete surgical resection remains the only option for cure with an estimated median survival ranging from 27 to 36 months ().Citation5,Citation7–Citation10
Figure 1 Types of cholangiocarcinoma.
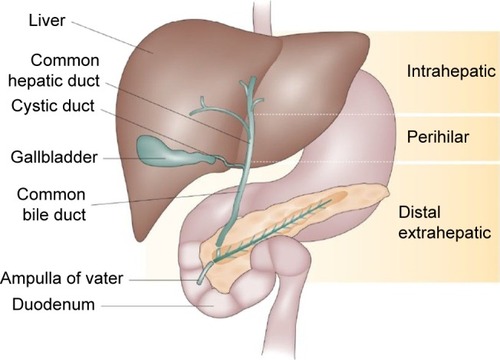
Figure 2 Overall survival in a large cohort of intrahepatic cholangiocarcinoma patients.
Abbreviation: AJCC, American Joint Committee on Cancer Staging.
Over three-quarters of patients are older than 65 years at initial diagnosis,Citation3 and ICC is slightly more common in men.Citation11 ICC is more common in East Asia; in the People’s Republic of China, an incidence of 10 per 100,000 persons has been reported, while in Thailand, the incidence is 71 per 100,000, higher than for hepatocellular carcinoma (HCC).Citation1,Citation12
In general, ICC has similar risk factors to HCC. A correlation with diseases causing biliary inflammation and fibrosis, such as primary sclerosing cholangitis and primary biliary cirrhosis, has been noted.Citation13,Citation14 Other risk factors for ICC are congenital malformations of the bile duct (ie, choledochal cysts), hepatolithiasis, hepatitis B and C virus, alcoholic liver cirrhosis, and smoking.Citation13 In East Asia, hepatic parasite infections, in particular Opisthorchis viverrini and Clonorchis sinensis, are significant risk factors.Citation15,Citation16 The reason for the vast difference in incidence between the east and west is not fully understood, as it cannot be attributed completely to the spread of the infectious risk factors.Citation1,Citation12
Histology
ICC mostly develops as a well-differentiated adenocarcinoma.Citation17,Citation18 Its formation is frequently caused by mutations of the KRAS oncogene, a protein normally involved in the cell proliferation, in combination with the deletion of the p53 tumor suppressor gene.Citation19 A critical signaling protein downstream of KRAS and p53 mutations is interleukin (IL) 6, which is a serum biomarker for ICC.Citation20–Citation22 Further downstream, ROS1 fusion proteins, regulated by KRAS/IL-6 pathways, have been associated with an aggressive phenotype and metastatic disease at diagnosis.Citation23,Citation24
Based on their histological appearance, ICCs can be divided into three histological growth types: the mass-forming, intraductal infiltrating, and periductal pattern.Citation25,Citation26 The most common of these growth patterns is the mass-forming pattern, of which the clinical symptoms may be similar to HCC as both involve the formation of a mass in the liver.Citation27,Citation28 On imaging (ie, computed tomography [CT] and magnetic resonance imaging [MRI]), these tumors are clearly visible and well delineated.Citation26 Mass-forming ICC typically has a diameter of 5–10 cm at the time of diagnosis.Citation29,Citation30 Intraductal ICC is a slowly growing papillary tumor and has a favorable prognosis compared with the other two types.Citation26 On imaging, it is a 1–2 cm mass within the bile duct with proximal ductal dilatation. The mass is usually confined to the bile duct wall.Citation26,Citation31,Citation32 Periductal infiltrating cholangiocarcinoma is characterized by growth along the bile duct without mass formation, which radiologically presents as a small lesion or diffuse bile duct thickening.Citation33 This type of tumor is a rare form of ICC and is commonly seen in combination with mass-forming ICC.Citation34,Citation35 The different histological appearances of cholangiocarcinoma necessitate different surgical strategies, since tumors growing along the bile duct (intraductal and periductal ICC) often require extrahepatic bile duct resection in addition to hepatic resection.Citation26,Citation36
ICC and HCC may occur simultaneously in the same patient or even in the same lesion.Citation37,Citation38 Combined HCC and ICC tumors mostly follow the more aggressive behavior of ICC.Citation37 Because of similar allelic losses in both HCC-like and ICC-like cells, these tumors are thought to have a monoclonal origin with bidirectional phenotype differentiation.Citation38,Citation39 In concordance with this hypothesis, a Korean group recently suggested that the acquisition of ICC characteristics is a leading cause of atypically aggressive HCC behavior.Citation40 Further research in the fields of imaging and molecular analysis is required to improve early diagnosis.Citation38
Staging
The most commonly used classification system to qualify advancement and resectability of ICC is the American Joint Committee on Cancer (AJCC) TNM staging system, currently in its seventh edition, consisting of four stages ().Citation41 Prior to this edition, there was no separate staging system for ICC, and these tumors were classified with HCC.Citation42 The T-stage is determined by the number of liver tumors, the presence of vascular invasion, and direct extrahepatic invasion. The T4 stage is reserved for tumors with a periductal growth pattern. N1 indicates the presence of regional lymph node metastases, and M1 indicates distant metastases.Citation42 Recent research suggests the AJCC staging system performs poorly in differentiating between various prognoses, with vast inter-patient survival differences within TNM stages.Citation43,Citation44 Additional independent prognostic factors have been identified to improve staging, including elevated serum carbohydrate antigen (CA) 19-9 and carcinoembryonic antigen (CEA), lympho(neuro)vascular invasion, and serum alkaline phosphatase (ALP).Citation44
Table 1 American Joint Committee on Cancer TNM classification, seventh edition
A genomic biomarker profile can also help in differentiating patients with ICC.Citation45–Citation47 A genomic study of 149 patients with ICC identified two molecular subgroups, an inflammation and a proliferation group, with distinct clinical outcomes. The inflammation subclass (40%) showed increased activation of inflammation pathways, overexpression of IL-6, IL-10, and IL-17, and constitutive activation of immune system transcription factor STAT3.Citation47,Citation48 The proliferation subclass (60%) showed increased activation of oncogenic pathways RAS/MAPK and MET, specific DNA mutations, and risk factors for poor clinical outcome.Citation13,Citation48
In a recent meta-analysis, we identified several immunohistochemistry biomarkers for patients with ICC.Citation45 An example of a diagnostic and prognostic biomarker is fascin, an actin cross-linked protein found in the cell membrane of the biliary duct cells.Citation49 The epidermal growth factor receptor also plays an important role in prognostics and is a potential treatment target.Citation50,Citation51 Mucin 1, cell surface associated and Mucin 4, cell surface associated are two membrane proteins that have been shown to be associated with patient prognosis.Citation52–Citation54 Lastly, p27, cyclin-dependent kinase inhibitor 1B, is a protein involved in the cell cycle, which also has predictive capabilities in relation to postoperative survival.Citation55–Citation57 In addition to these biomarkers, several other biomarkers have been shown to have an impact on diagnostics, prognostics, and treatment efficacy: HSP27; Akt; HDGF; Mucin 6, cell surface-associated; p16; p-4EBP1; S100A4; alpha-SMA; keratin 903; and TROP2.Citation45 A composite biomarker profile could improve prognosis and guide treatment selection.Citation47
Diagnosis and preoperative workup
The initial diagnosis of ICC is mostly made when the tumor is not eligible for resection because of locally advanced or metastatic disease.Citation13,Citation14,Citation58 Typically, a very large mass has developed in the periphery of the liver with few clinical symptoms.Citation19 Most patients present with nonspecific symptoms, such as pain in the right upper abdominal quadrant, weight loss, and high serum ALP levels. Some patients present with painless jaundice, when the tumor grows towards the biliary confluence.Citation14,Citation58 Small ICCs are found in screening programs for early detection of HCC.Citation59
Transabdominal ultrasound is often the first imaging modality that detects a liver mass with or without dilatation of the biliary tract.Citation60 The number of lesions and vascular involvement are determined using a dual-phase multi-detector CT. Typical appearance of ICC on CT is a hypodense mass with irregular margins on unenhanced scans, peripheral rim enhancement in the arterial contrast-enhancement phase, and progressive contrast uptake in the (portal-)venous and delayed contrast-enhancement phase.Citation61 Small ICCs can be difficult to distinguish from HCC. Biliary drainage (if needed) should be performed after imaging because the presence of stents and drains hampers accurate assessment of the extent of the tumor.Citation62
Both magnetic resonance cholangiopancreatography (MRCP) and positron emission tomography (PET) have a good accuracy for diagnosis and assessment of the extent of the tumor. MRCP has a diagnostic accuracy of up to 93% and is recommended for visualization of the tumor extension in the ductal system and vascular structures.Citation47,Citation63 Clinical utility of PET for diagnosing ICC in the liver when CT or MRI has been performed is limited.Citation47 However, preoperative PET scanning may be considered to help rule out occult metastatic disease, as PET changes surgical decision making in up to 30% of patients.Citation64–Citation66 Despite these imaging modalities, as many as a third of patients with resectable disease on imaging have occult metastatic or locally advanced disease during diagnostic laparoscopy.Citation67,Citation68 Therefore, better imaging is needed to avoid surgery in these patients.Citation14,Citation67,Citation68
Biliary drainage and portal vein embolization
ICC may cause biliary obstruction when the tumor grows towards the liver hilum. Biliary drainage may be required in the preoperative setting with resectable disease and in the palliative setting. Biliary drainage aims to improve liver function and increase appetite.Citation69 Moreover, preoperative biliary drainage may improve liver regeneration and decrease the risk of postoperative liver failure.Citation70,Citation71 The main drawback of biliary drainage is colonization of the bile duct that often results in cholangitis.Citation72 Patients with a future liver remnant of at least 50% should probably undergo a resection without preoperative biliary drainage.Citation73,Citation74 Drainage can be performed endoscopically or percutaneously. Biliary drainage can reduce symptoms and improve quality of life in the palliative setting.Citation75,Citation76
A resection of more than 75% of the total liver volume in a healthy liver and more than 65% of the total liver volume in a compromised liver (eg, due to cirrhosis or fibrosis) is an indication of portal vein embolization (PVE).Citation77 PVE results in hypertrophy of the future liver remnant by pre-operatively embolizing the liver that will be resected.Citation77 In a total of 1,791 patients with different hepatic tumors, PVE had a technical success of 96.1%.Citation77
Surgical management
Resection
Surgical treatment is the only potentially curative treatment in patients with ICC. ICC is an aggressive cancer, when compared to other primary hepatic neoplasms.Citation4,Citation14,Citation58 A large study (n=584) demonstrated that even after curative-intent resection, the probability of cure is only about 10%.Citation78 Because of the large size as well as intraductal and periductal spread, major hepatectomies are required to obtain negative resection margins.Citation4 With regard to prognosis, resection is only useful when a complete resection (R0) with negative resection margins is anticipated. Moreover, the liver remnant should be adequate in size and function, with or without prior PVE.Citation8,Citation77,Citation79,Citation80 Extrahepatic disease, including lymph node metastases beyond the regional basin (N2), is a contraindication for curative-intent surgery.Citation41 Multifocal ICC is considered unresectable by some experts.Citation79–Citation83 Nevertheless, other experts report favorable long-term outcomes in selected patients with typically two to three lesions, with a 5-year overall survival (OS) of 20%.Citation84,Citation85 A 2015 cure model confirms the possibility of cure, albeit at a chance of only 4%.Citation78 Recent studies have reported favorable outcomes of portal vein reconstructions.Citation86–Citation88 However, tumor invasion of the main hepatic artery and bilateral hepatic artery involvement remain contraindications for resection in most Western centers. Hepatic artery reconstruction is associated with a high risk of postoperative mortality as well as poor oncologic outcomes.Citation89,Citation90
A complete resection of ICC involves an (extended) hemihepatectomy in most (75%) of patients. Many patients (25%) also require a bile duct resection and reconstruction. Morbidity rates are often more than one in five, and mortality rates vary from 1% to 6%.Citation8,Citation9,Citation91 Intraoperative and postoperative strategies, such as low central venous pressure, restricted fluid resuscitation, and enhanced recovery pathways, have improved recovery and decreased the risk of complications.Citation87,Citation88,Citation92 A recent article reviewed perioperative management of patients undergoing hepatic resection.Citation93 The authors noted that surgeons left an operative drain in almost half of patients undergoing liver resection, even though most data suggest that routine operative drainage after liver resection (without a biliary anastomosis) is unnecessary and should generally be avoided.Citation94–Citation96
Whereas HCC is commonly treated with orthotopic liver transplantation (OLT), ICC as an indication for OLT is still controversial.Citation97 Historical evidence suggests poor outcomes for ICC in single-center studies.Citation98–Citation104 Outcomes of OLT for combined HCC and ICC were also predominantly unfavorable.Citation98,Citation105 Five-year survival estimates in these studies ranged from 10% to 18%, which is clearly inferior to the benchmark of OLT of about 70%.Citation97 More recent studies indicate that strictly selected patients might benefit from OLT, particularly patients with ICC smaller than 2 cm.Citation106
Systemic chemotherapy
Preoperative chemotherapy
Preoperative chemotherapy (pCT) can be administered for multiple purposes, although it is not routinely prescribed due to a lack of evidence.Citation107 Neoadjuvant therapy is employed to address occult metastatic disease or facilitate resection. We recently evaluated the role of pCT in a cohort of 1,057 patients, of whom 62 patients received chemotherapy. We found that patients receiving pCT had similar survival following curative-intent resection, regardless of more advanced disease.Citation107 No regimen is currently proven to have effect during the preoperative period. In light of the outcomes of the ABC-02 trial, discussed later, a combination of gemcitabine and cisplatin was offered most often.Citation108
Adjuvant chemotherapy
Adjuvant chemotherapy is aimed at decreasing the chance of tumor recurrence.Citation109 Chemotherapy consists of mainly nucleoside analogs, most commonly gemcitabine, sometimes in combination with cisplatin.Citation109 Systemic therapy is known to have a large impact on patient’s quality of life, and form a large financial burden. The efficacy of chemotherapy regimens in ICC is usually poor, with only a small subgroup benefitting significantly in both quality of life and length of survival.Citation16,Citation109 While a significant portion of the US patients receive chemotherapy, no randomized trials have been completed.Citation42 A multicenter phase III trial is currently accruing patients to determine the effectiveness of adjuvant gemcitabine and cisplatin in patients with biliary cancer ().
Table 2 Currently active phase III and phase IV studies
Palliative chemotherapy
A phase III trial, the ABC-02 trial, randomized 410 patients with biliary cancer (ie, cholangiocarcinoma and gallbladder cancer) and found an improvement in OS of nearly 4 months with gemcitabine plus cisplatin compared to gemcitabine alone.Citation108 A combined analysis of the ABC-02 trial and the Japanese BT22 trial, conducted in a comparable setting, found a hazard ratio of 0.54 (95% confidence interval 0.36–0.81) for the subgroup of 108 patients with ICC.Citation110 Gemcitabine plus cisplatin has been the standard palliative regimen for locally advanced or metastatic ICC since. Best supportive care is recommended for patients with a poor performance status or a life expectancy of less than 6 months.Citation111–Citation114
Regional treatments
Regional treatments rely on the dual blood supply of the liver, where the hepatic artery is mostly responsible for the blood supply of tumors, as illustrated by early arterial enhancement on imaging.Citation115–Citation117 Hepatic arterial infusion (HAI) chemotherapy using a subcutaneous pump has been investigated for patients with ICC at Memorial Sloan Kettering Cancer Center (MSKCC). It involves continuous infusion of floxuridine directly into the hepatic artery. Intra-arterial delivery allows for a 200-fold higher drug delivery to the tumor with little systemic toxicity because of the 95% first-pass effect of floxuridine in the liver.Citation5 HAI chemotherapy has been studied extensively in common malignancies, such as colorectal liver metastases.Citation5,Citation118
In a recent study from MSKCC, HAI with floxuridine was combined with systemic chemotherapy in patients with locally advanced (ie, unresectable without extrahepatic disease) ICC (n=104).Citation5 Outcomes were compared with locally advanced patients receiving systemic chemotherapy alone.Citation5 Median OS was superior with HAI chemotherapy (30.8 months vs 18.4 months; P<0.001). Five-year OS was 20% in patients who received HAI chemotherapy compared with 5% in the systemic-only group. In comparison, 5-year OS was 0% in the ABC-02 trial.Citation108 Moreover, the partial response rate (RECIST criteria) in the HAI chemotherapy group was 59%, with conversion to resectability in eight of 104 patients (13%). Future prospective studies should be conducted in order to confirm these results. Currently, a phase II trial is recruiting patients for HAI chemotherapy in the adjuvant setting (NCT01312857).
Other hepatic artery-based treatments for locally advanced ICC include transarterial chemoembolization (TACE) and radio-embolization with yttrium-90 (Y-90).Citation115 TACE affects the blood flow to the tumor in addition to locally releasing cytotoxic agents. It causes ischemic tumor necrosis and facilitates intracellular transit of chemotherapeutic agents.Citation115,Citation117 In a study of 41 prospectively followed patients, one group described a median OS of 11.7 months from first treatment, after treatment with irinotecan TACE.Citation119 One patient successfully underwent resection following TACE.Citation119 Another prospective study reported a median survival of 17.5 months in 24 patients, with three patients being adequately downstaged to undergo resection.Citation120 Despite the encouraging results, no phase III trial has been performed.Citation115
Y-90 radio-embolization therapy also aims to improve life expectancy in patients with unresectable HCC and colorectal liver metastases.Citation115 The technique is based on administration of beads filled with the radioactive isotope yttrium Y-90 microspheres into the hepatic artery branch responsible for the lobes of the liver beset by tumor.Citation121,Citation122 Prior to treatment, embolization of the nontarget vessels and injection of technetium-99mm-labeled macro-aggregated albumin is performed, in order to exclude extrahepatic accumulation.Citation115,Citation121,Citation122 Several small studies indicate that Y-90 is tolerated well in patients with a good performance status.Citation123–Citation128 In ICC patients, Y-90 was associated with improved survival, when compared with patients undergoing best supportive care only.Citation123–Citation128 Estimates ranged from 9 months posttreatment in a cohort of 25 Australian patients,Citation127 to 22 months in a cohort of 33 German patients.Citation126 Randomized trials are required to determine the effectiveness of Y-90 therapy.
Prognostic models and nomograms
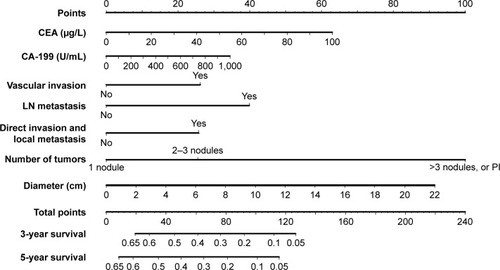
Several prognostic models have been developed in addition to the AJCC staging. More accurate prediction of individual patient outcome may provide better individual survival estimates, as well as improve identification of high-risk groups who may benefit from adjuvant therapy.Citation11 While the AJCC staging concerns all ICC patients, other models pertain only to patients who have undergone a complete resection. A Chinese nomogram predicts individual OS after resection of ICC ().Citation43 Prognostic factors in this model included CEA, CA19-9, vascular invasion, presence of lymph node metastases, direct invasion and local metastases, number of tumors, and tumor diameter. A similar model was developed with a multinational dataset without tumor markers. Risk factors for survival after resection were age, number of tumors, tumor diameter, cirrhosis, lymph node metastases, and macrovascular invasion.Citation129 The Chinese nomogram had superior discrimination at external validation.Citation43,Citation44
Figure 3 Validated intrahepatic cholangiocarcinoma nomogram predicting overall survival. Adapted from Wang et al.Citation43
Abbreviations: CEA, carcino-embryonic antigen; LN, lymph node; PI, periductal invasion.
Other prognostic models were developed for conditional survival, accounting for the years that a patient had already survived after surgery.Citation84,Citation130,Citation131 Conditional survival was found to be the most important prognostic factor, when predicting future survival time.Citation84,Citation130,Citation131 OS in this study decreased over time to 16% at 8 years, while the 3-year conditional survival at 5 years, that is, the chance of surviving to year 8 after having survived to year 5, was 65%.Citation84
Personalized treatments
Personalized treatments for ICC patients could improve the overall outcomes, mainly by withholding treatments from patients who are unlikely to benefit from surgery or chemotherapy. For example, patients with a very poor predicted survival after surgery (eg, 3-year OS below 5% based on the Chinese nomogram in ) are unlikely to benefit from surgery. Unfortunately, predictive biomarkers for response to systemic chemotherapy are not available.Citation45 Future studies should further improve prognostic models and identify predictive biomarkers to determine the response to chemotherapy.Citation44,Citation132
Future perspectives
ICC is a complex disease, with a dismal prognosis. ICC is typically diagnosed with metastatic or locally advanced disease. Surgery may improve both survival and quality of life, but comes with a substantial risk of postoperative morbidity and mortality. The benefit of palliative systemic treatment is real but small. The merits of (neo)adjuvant therapy still need to be explored in phase III trials. Targeted therapies (eg, targeting IDH 1 or 2 mutations) are promising but require further evaluation.Citation133 HAI, TACE, and radio-embolization are promising locoregional techniques. Appropriate allocation of all locoregional and systemic treatments may further improve with better knowledge of histopathology and biological behavior.
Ideally, low-cost diagnostic biomarkers could reliably detect ICC in patients presenting with vague symptoms of the upper abdomen or screened for liver cancer. Furthermore, predictive biomarkers are required to determine in advance which patients will benefit from chemotherapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- ShinHROhJKMasuyerEComparison of incidence of intrahepatic and extrahepatic cholangiocarcinoma – focus on East and South-Eastern AsiaAsian Pac J Cancer Prev20101151159116621198257
- SingalAKVautheyJNGradyJJStroehleinJRIntra-hepatic cholangiocarcinoma – frequency and demographic patterns: thirty-year data from the M.D. Anderson Cancer CenterJ Cancer Res Clin Oncol201113771071107821207060
- EverhartJERuhlCEBurden of digestive diseases in the United States Part III: liver, biliary tract, and pancreasGastroenterology200913641134114419245868
- DeOliveiraMLCunninghamSCCameronJLCholangiocarcinoma: thirty-one-year experience with 564 patients at a single institutionAnn Surg2007245575576217457168
- KonstantinidisITGroot KoerkampBDoRKUnresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy aloneCancer2016122575876526695839
- EsnaolaNFMeyerJEKarachristosAMarankiJLCampERDenlingerCSEvaluation and management of intrahepatic and extrahepatic cholangiocarcinomaCancer201612291349136926799932
- NakeebATranKQBlackMJImproved survival in resected biliary malignanciesSurgery20021324555563 discussion 563–55412407338
- EndoIGonenMYoppACIntrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resectionAnn Surg20082481849618580211
- de JongMCNathanHSotiropoulosGCIntrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessmentJ Clin Oncol201129233140314521730269
- AminiNEjazASpolveratoGKimYHermanJMPawlikTMTemporal trends in liver-directed therapy of patients with intrahepatic cholangiocarcinoma in the United States: a population-based analysisJ Surg Oncol2014110216317024676600
- TysonGLEl-SeragHBRisk factors for cholangiocarcinomaHepatology201154117318421488076
- KhanSAToledanoMBTaylor-RobinsonSDEpidemiology, risk factors, and pathogenesis of cholangiocarcinomaHPB (Oxford)2008102778218773060
- BritoAFAbrantesAMEncarnacaoJCTralhãoJGBotelhoMFCholangiocarcinoma: from molecular biology to treatmentMed Oncol2015321124526427701
- DodsonRMWeissMJCosgroveDIntrahepatic cholangiocarcinoma: management options and emerging therapiesJ Am Coll Surg20132174736750.e423890842
- CasperFWSeufertRJAtrial natriuretic peptide (ANP) in preeclampsia-like syndrome in a rat modelExp Clin Endocrinol Diabetes199510352922968536057
- AndersonCDPinsonCWBerlinJChariRSDiagnosis and treatment of cholangiocarcinomaOncologist2004914357
- OlnesMJErlichRA review and update on cholangiocarcinomaOncology200466316717915218306
- NakanumaYSatoYHaradaKSasakiMXuJIkedaHPathological classification of intrahepatic cholangiocarcinoma based on a new conceptWorld J Hepatol201021241942721191517
- O’DellMRHuangJLWhitney-MillerCLKras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinomaCancer Res20127261557156722266220
- FavaGLorenziniIMolecular pathogenesis of cholangiocarcinomaInt J Hepatol2012201263054321994887
- JohnsonCHanYHughartNMcCarraJAlpiniGMengFInterleukin-6 and its receptor, key players in hepatobiliary inflammation and cancerTransl Gastrointest Cancer201211587022724089
- GoydosJSBrumfieldAMFrezzaEBoothALotzeMTCartySEMarked elevation of serum interleukin-6 in patients with cholangiocarcinoma: validation of utility as a clinical markerAnn Surg199822733984049527063
- LeeKHLeeKBKimTYClinical and pathological significance of ROS1 expression in intrahepatic cholangiocarcinomaBMC Cancer20151572126475437
- DengGHuCZhuLDownregulation of ROS-FIG inhibits cell proliferation, colonyformation, cell cycle progression, migration and invasion, while inducing apoptosis in intrahepatic cholangiocarcinoma cellsInt J Mol Med201434366166824968753
- LazaridisKNGoresGJCholangiocarcinomaGastroenterology200512861655166715887157
- ChungYEKimMJParkYNVarying appearances of cholangiocarcinoma: radiologic-pathologic correlationRadiographics200929368370019448110
- KangYLeeJMKimSHHanJKChoiBIIntrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR imagesRadiology2012264375176022798225
- OkabayashiTYamamotoJKosugeTA new staging system for mass-forming intrahepatic cholangiocarcinoma: analysis of preoperative and postoperative variablesCancer20019292374238311745293
- KimSJLeeJMHanJKKimKHLeeJYChoiBIPeripheral mass-forming cholangiocarcinoma in cirrhotic liverAJR Am J Roentgenol200718961428143418029881
- MamoneGMarroneGCarusoSIntrahepatic mass-forming cholangiocarcinoma: enhancement pattern on Gd-BOPTA-MRI with emphasis of hepatobiliary phaseAbdom Imaging20154072313232225962708
- LimJHCholangiocarcinoma: morphologic classification according to growth pattern and imaging findingsAJR Am J Roentgenol2003181381982712933488
- LimJHYiCALimHKLeeWJLeeSJKimSHRadiological spectrum of intraductal papillary tumors of the bile ductsKorean J Radiol200231576311919480
- MittelstaedtCAUltrasound of the bile ductsSemin Roentgenol19973231611719253067
- ParkHSLeeJMKimSHCT differentiation of cholangiocarcinoma from periductal fibrosis in patients with hepatolithiasisAJR Am J Roentgenol2006187244545316861550
- LimJHParkCKPathology of cholangiocarcinomaAbdom Imaging200429554054715383897
- SasakiAAramakiMKawanoKIntrahepatic peripheral cholangiocarcinoma: mode of spread and choice of surgical treatmentBr J Surg1998859120612099752860
- JarnaginWRWeberSTickooSKCombined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factorsCancer20029472040204611932907
- MaximinSGaneshanDMShanbhogueAKCurrent update on combined hepatocellular-cholangiocarcinomaEur J Radiol Open20141404826937426
- WuPCFangJWLauVKLaiCLLoCKLauJYClassification of hepatocellular carcinoma according to hepatocellular and biliary differentiation markers. Clinical and biological implicationsAm J Pathol19961494116711758863666
- WooHGLeeJHYoonJHIdentification of a cholangiocarcinoma-like gene expression trait in hepatocellular carcinomaCancer Res20107083034304120395200
- EdgeSBByrdDRComptonCCFritzAGGreeneFLTrottiAAJCC Cancer Staging Manual7th edParisSpringer2010
- WeberSMRiberoDO’ReillyEMKokudoNMiyazakiMPawlikTMIntrahepatic cholangiocarcinoma: expert consensus statementHPB (Oxford)201517866968026172134
- WangYLiJXiaYPrognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomyJ Clin Oncol20133191188119523358969
- DoussotAGroot-KoerkampBWiggersJKOutcomes after resection of intrahepatic cholangiocarcinoma: external validation and comparison of prognostic modelsJ Am Coll Surg2015221245246126206643
- RuysATGroot KoerkampBWiggersJKKlümpenHJten KateFJvan GulikTMPrognostic biomarkers in patients with resected cholangiocarcinoma: a systematic review and meta-analysisAnn Surg Oncol201421248750024081803
- WiggersJKRuysATGroot KoerkampBBeuersUten KateFJvan GulikTMDifferences in immunohistochemical biomarkers between intra- and extrahepatic cholangiocarcinoma: a systematic review and meta-analysisJ Gastroenterol Hepatol20142981582159424787096
- BridgewaterJGallePRKhanSAGuidelines for the diagnosis and management of intrahepatic cholangiocarcinomaJ Hepatol20146061268128924681130
- SiaDHoshidaYVillanuevaAIntegrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomesGastroenterology2013144482984023295441
- IguchiTAishimaSTaketomiAFascin overexpression is involved in carcinogenesis and prognosis of human intrahepatic cholangiocarcinoma: immunohistochemical and molecular analysisHum Pathol200940217418018835624
- ShafizadehNGrenertJPSahaiVKakarSEpidermal growth factor receptor and HER-2/neu status by immunohistochemistry and fluorescence in situ hybridization in adenocarcinomas of the biliary tree and gallbladderHum Pathol201041448549220040392
- HerbergerBBergerWPuhallaHSimultaneous blockade of the epidermal growth factor receptor/mammalian target of rapamycin pathway by epidermal growth factor receptor inhibitors and rapamycin results in reduced cell growth and survival in biliary tract cancer cellsMol Cancer Ther2009861547155619509244
- HigashiMYamadaNYokoyamaSPathobiological implications of MUC16/CA125 expression in intrahepatic cholangiocarcinoma-mass forming typePathobiology201279210110622286058
- ParkSYRohSJKimYNExpression of MUC1, MUC2, MUC5AC and MUC6 in cholangiocarcinoma: prognostic impactOncol Rep200922364965719639217
- YehCNPangSTWuRCChenTWJanYYChenMFPrognostic value of MUC4 for mass-forming intrahepatic cholangiocarcinoma after hepatectomyOncol Rep2009211495619082442
- HashimotoNYachidaSOkanoKImmunohistochemically detected expression of p27(Kip1) and Skp2 predicts survival in patients with intrahepatic cholangiocarcinomasAnn Surg Oncol200916239540319034576
- JarnaginWRKlimstraDSHezelMDifferential cell cycle- regulatory protein expression in biliary tract adenocarcinoma: correlation with anatomic site, pathologic variables, and clinical outcomeJ Clin Oncol20062471152116016505435
- FiorentinoMAltimariAD’ErricoALow p27 expression is an independent predictor of survival for patients with either hilar or peripheral intrahepatic cholangiocarcinomaClin Cancer Res20017123994399911751492
- RazumilavaNGoresGJCholangiocarcinomaLancet201438399352168217924581682
- BrownKMParmarADGellerDAIntrahepatic cholangiocarcinomaSurg Oncol Clin N Am201423223124624560108
- Netherlands Comprehensive Cancer OrganisationOncoline: Clinical practice guidelines Available from: http://www.oncoline.nl/Accessed August 10, 2016
- VallsCGumaAPuigIIntrahepatic peripheral cholangiocarcinoma: CT evaluationAbdom Imaging200025549049610931983
- WeberASchmidRMPrinzCDiagnostic approaches for cholangiocarcinomaWorld J Gastroenterol200814264131413618636656
- SutharMPurohitSBhargavVGoyalPRole of MRCP in differentiation of benign and malignant causes of biliary obstructionJ Clin Diagn Res2015911TC08TC12
- AndersonCDRiceMHPinsonCWChapmanWCChariRSDelbekeDFluorodeoxyglucose PET imaging in the evaluation of gallbladder carcinoma and cholangiocarcinomaJ Gastrointest Surg200481909714746840
- KimYJYunMLeeWJKimKSLeeJDUsefulness of 18F-FDG PET in intrahepatic cholangiocarcinomaEur J Nucl Med Mol Imaging200330111467147214579085
- CorveraCUBlumgartLHAkhurstT18F-fluorodeoxyglucose positron emission tomography influences management decisions in patients with biliary cancerJ Am Coll Surg20082061576518155569
- WeberSMJarnaginWRKlimstraDDeMatteoRPFongYBlumgartLHIntrahepatic cholangiocarcinoma: resectability, recurrence pattern, and outcomesJ Am Coll Surg2001193438439111584966
- GoereDWagholikarGDPessauxPUtility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinomaSurg Endosc200620572172516508808
- van der GaagNAKloekJJde CastroSMBuschORvan GulikTMGoumaDJPreoperative biliary drainage in patients with obstructive jaundice: history and current statusJ Gastrointest Surg200913481482018726134
- NimuraYPreoperative biliary drainage before resection for cholangiocarcinoma (Pro)HPB (Oxford)200810213013318773090
- KawasakiSImamuraHKobayashiANoikeTMiwaSMiyagawaSResults of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemi-hepatic portal vein embolizationAnn Surg20032381849212832969
- IaconoCRuzzenenteACampagnaroTBortolasiLValdegamberiAGuglielmiARole of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacksAnn Surg2013257219120423013805
- WiggersJKGroot KoerkampBCieslakKPPostoperative mortality after liver resection for perihilar cholangiocarcinoma: development of a risk score and importance of biliary drainage of the future liver remnantJ Am Coll Surg20162232321331.e127063572
- FargesORegimbeauJMFuksDMulticentre European study of preoperative biliary drainage for hilar cholangiocarcinomaBr J Surg2013100227428323124720
- van DeldenOMLamérisJSPercutaneous drainage and stenting for palliation of malignant bile duct obstructionEur Radiol200818344845617960388
- GamanagattiSSinghTSharmaRSrivastavaDNDashNRGargPKUnilobar versus bilobar biliary drainage: effect on quality of life and bilirubin level reductionIndian J Palliat Care2016221506226962281
- van LiendenKPvan den EsschertJWde GraafWPortal vein embolization before liver resection: a systematic reviewCardiovasc Intervent Radiol2013361253422806245
- SpolveratoGVitaleACucchettiACan hepatic resection provide a long-term cure for patients with intrahepatic cholangiocarcinoma?Cancer2015121223998400626264223
- RiberoDPinnaADGuglielmiASurgical approach for long-term survival of patients with intrahepatic cholangiocarcinoma: a multi-institutional analysis of 434 patientsArch Surg2012147121107111322910846
- HyderOHatzarasISotiropoulosGCRecurrence after operative management of intrahepatic cholangiocarcinomaSurgery2013153681181823499016
- LuoXYuanLWangYGeRSunYWeiGSurvival outcomes and prognostic factors of surgical therapy for all potentially resectable intrahepatic cholangiocarcinoma: a large single-center cohort studyJ Gastrointest Surg201418356257224395070
- SulpiceLRayarMBoucherEPrachtMMeunierBBoudjemaKTreatment of recurrent intrahepatic cholangiocarcinomaBr J Surg201299121711171723132419
- FargesOFuksDBoleslawskiEInfluence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study groupAnn Surg20112545824829 discussion 83022042474
- SpolveratoGKimYEjazAConditional probability of long-term survival after liver resection for intrahepatic cholangiocarcinoma: a multi-institutional analysis of 535 patientsJAMA Surg2015150653854525831462
- SpolveratoGKimYAlexandrescuSIs hepatic resection for large or multifocal intrahepatic cholangiocarcinoma justified? Results from a multi-institutional collaborationAnn Surg Oncol20152272218222525354576
- AminiNSpolveratoGKimYPawlikTMTrends in hospital volume and failure to rescue for pancreatic surgeryJ Gastrointest Surg20151991581159225794484
- GurusamyKSLiJVaughanJSharmaDDavidsonBRCardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resectionCochrane Database Syst Rev20125CD00733822592720
- KimYEjazAGaniFCrystalloid administration among patients undergoing liver surgery: defining patient- and provider-level variationSurgery2016159238939826263833
- HartogHIjzermansJNvan GulikTMGroot KoerkampBResection of perihilar cholangiocarcinomaSurg Clin North Am201696224726727017863
- AbbasSSandroussiCSystematic review and meta-analysis of the role of vascular resection in the treatment of hilar cholangiocarcinomaHPB (Oxford)201315749250323750491
- GiulianteFGauzolinoRVelloneMArditoFMurazioMNuzzoGLiver resection for intrahepatic cholangiocarcinomaTumori200591648749216457147
- GurusamyKSLiJSharmaDDavidsonBRCardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resectionCochrane Database Syst Rev20094CD00733819821405
- SpolveratoGEjazAKimYPatterns of care among patients undergoing hepatic resection: a query of the National Surgical Quality Improvement Program-targeted hepatectomy databaseJ Surg Res2015196222122825881789
- FongYBrennanMFBrownKHeffernanNBlumgartLHDrainage is unnecessary after elective liver resectionAm J Surg199617111581628554132
- ButteJMGrendarJBatheOThe role of peri-hepatic drain placement in liver surgery: a prospective analysisHPB (Oxford)2014161093694225041265
- Brooke-SmithMFiguerasJUllahSProspective evaluation of the International Study Group for Liver Surgery definition of bile leak after a liver resection and the role of routine operative drainage: an international multicentre studyHPB (Oxford)2015171465125059275
- SapisochinGFernández de SevillaEEcheverriJCharcoRLiver transplantation for cholangiocarcinoma: current status and new insightsWorld J Hepatol20157222396240326464755
- DeOliveiraMLLiver transplantation for cholangiocarcinoma: current best practiceCurr Opin Organ Transplant201419324525224811436
- RoblesRFiguerasJTurrionVSSpanish experience in liver transplantation for hilar and peripheral cholangiocarcinomaAnn Surg2004239226527114745336
- CasavillaFAMarshJWIwatsukiSHepatic resection and transplantation for peripheral cholangiocarcinomaJ Am Coll Surg199718554294369358085
- JanYYYehCNYehTSChenTCPrognostic analysis of surgical treatment of peripheral cholangiocarcinoma: two decades of experience at Chang Gung Memorial HospitalWorld J Gastroenterol200511121779178415793863
- MeyerCGPennIJamesLLiver transplantation for cholangiocarcinoma: results in 207 patientsTransplantation20006981633163710836374
- GhaliPMarottaPJYoshidaEMLiver transplantation for incidental cholangiocarcinoma: analysis of the Canadian experienceLiver Transpl200511111412141616237695
- WeimannAVarnholtHSchlittHJRetrospective analysis of prognostic factors after liver resection and transplantation for cholangiocellular carcinomaBr J Surg20008791182118710971425
- GroeschlRTPappasSGChristiansKKAre we justified in excluding combined hepatocellular-cholangiocarcinoma from transplantation?J Clin Oncol201230Suppl 425622184397
- RizviSGoresGJPathogenesis, diagnosis, and management of cholangiocarcinomaGastroenterology201314561215122924140396
- BuettnerSGroot KoerkampBEjazAThe effect of preoperative chemotherapy treatment in surgically treated intrahepatic cholangiocarcinoma patients – a multi-institutional analysisJ Surg Oncol2016
- ValleJWasanHPalmerDHABC-02 Trial InvestigatorsCisplatin plus gemcitabine versus gemcitabine for biliary tract cancerN Engl J Med2010362141273128120375404
- HorganAMAmirEWalterTKnoxJJAdjuvant therapy in the treatment of biliary tract cancer: a systematic review and meta-analysisJ Clin Oncol201230161934194022529261
- ValleJWFuruseJJitlalMCisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trialsAnn Oncol201425239139824351397
- GoenkaMKGoenkaUPalliation: hilar cholangiocarcinomaWorld J Hepatol20146855956925232449
- TaylorMCMcLeodRSLangerBBiliary stenting versus bypass surgery for the palliation of malignant distal bile duct obstruction: a meta-analysisLiver Transpl20006330230810827230
- SmithACDowsettJFRussellRCHatfieldARCottonPBRandomised trial of endoscopic stenting versus surgical bypass in malignant low bileduct obstructionLancet19943448938165516607996958
- AndersenJRSørensenSMKruseARokkjaerMMatzenPRandomised trial of endoscopic endoprosthesis versus operative bypass in malignant obstructive jaundiceGut1989308113211352475392
- SeidenstickerRRickeJSeidenstickerMIntegration of chemoembolization and radioembolization into multimodal treatment of cholangiocarcinomaBest Pract Res Clin Gastroenterol201529231933225966431
- BreedisCYoungGThe blood supply of neoplasms in the liverAm J Pathol195430596997713197542
- LlovetJMRealMIMontanaXArterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trialLancet200235993191734173912049862
- McAuliffeJCQadanMD’AngelicaMIHepatic resection, hepatic arterial infusion pump therapy, and genetic biomarkers in the management of hepatic metastases from colorectal cancerJ Gastrointest Oncol20156669970826697204
- KuhlmannJBEuringerWSpangenbergHCTreatment of unresectable cholangiocarcinoma: conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapyEur J Gastroenterol Hepatol201224443744322261548
- SchiffmanSCMetzgerTDubelGPrecision hepatic arterial irinotecan therapy in the treatment of unresectable intrahepatic cholangiocellular carcinoma: optimal tolerance and prolonged overall survivalAnn Surg Oncol201118243143820862554
- SeidenstickerRDeneckeTKrausPMatched-pair comparison of radioembolization plus best supportive care versus best supportive care alone for chemotherapy refractory liver-dominant colorectal metastasesCardiovasc Intervent Radiol20123551066107321800231
- SangroBCarpaneseLCianniREuropean Network on Radioembolization with Yttrium-90 Resin Microspheres (ENRY)Survival after yttrium-90 resin microsphere radioembolization of hepatocellular carcinoma across Barcelona clinic liver cancer stages: a European evaluationHepatology201154386887821618574
- CamachoJCKokabiNXingMPrajapatiHJEl-RayesBKimHSModified response evaluation criteria in solid tumors and European Association for the Study of the Liver criteria using delayed-phase imaging at an early time point predict survival in patients with unresectable intrahepatic cholangiocarcinoma following yttrium-90 radioembolizationJ Vasc Interv Radiol201425225626524461131
- MouliSMemonKBakerTYttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysisJ Vasc Interv Radiol20132481227123423602420
- RafiSPiduruSMEl-RayesBYttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety studyCardiovasc Intervent Radiol201336244044822956045
- HoffmannRTPaprottkaPMSchönATransarterial hepatic yttrium-90 radioembolization in patients with unresectable intrahepatic cholangiocarcinoma: factors associated with prolonged survivalCardiovasc Intervent Radiol201235110511621431970
- SaxenaABesterLChuaTCChuFCMorrisDLYttrium-90 radiotherapy for unresectable intrahepatic cholangiocarcinoma: a preliminary assessment of this novel treatment optionAnn Surg Oncol201017248449119876691
- IbrahimSMMulcahyMFLewandowskiRJTreatment of unresectable cholangiocarcinoma using yttrium-90 microspheres: results from a pilot studyCancer200811382119212818759346
- HyderOMarquesHPulitanoCA nomogram to predict long-term survival after resection for intrahepatic cholangiocarcinoma: an Eastern and Western experienceJAMA Surg2014149543243824599477
- KimYEjazASpolveratoGConditional survival after surgical resection of gastric cancer: a multi-institutional analysis of the US Gastric Cancer CollaborativeAnn Surg Oncol201522255756425287440
- NathanHde JongMCPulitanoCConditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patientsJ Am Coll Surg2010210575576476476620421045
- Groot KoerkampBFongYOutcomes in biliary malignancyJ Surg Oncol2014110558559125250887
- MerlaALiuKGRajdevLTargeted therapy in biliary tract cancersCurr Treat Options Oncol201516104826266637
- BlechaczBKomutaMRoskamsTGoresGJClinical diagnosis and staging of cholangiocarcinomaNat Rev Gastroenterol Hepatol20118951252221808282
