Abstract
Background
Liver transplantation (LT) offers the most effective treatment for hepatocellular carcinoma patients. Various preoperative variables are correlated with survival after LT, but the prognostic role of aging on LT remains controversial.
Methods
Between January 2001 and December 2011, 290 consecutive transplants for patients with hepatocellular carcinoma performed in Shanghai First People’s Hospital (People’s Republic of China) were analyzed retrospectively. We compared patient characteristics and survival curves between a younger group (less than 49 years, n=135) and an aged group (50 years or older, n=155). We then performed Cox multivariate regression analysis of the risk factors for survival in aged and younger patients.
Results
Younger age was associated with higher alpha-fetoprotein (P=0.014), larger tumor size (P=0.038), poorer differentiation (P=0.025), portal lymph node metastasis (P=0.001), and higher recurrence rate (P=0.038). Aged patients had significantly longer recurrence-free survival and overall survival (P=0.020 and P=0.014, respectively); however, there were no significant differences between the younger and aged patients who met the Milan criteria (P>0.05). The 1-, 3-, and 5-year recurrence-free survival rates were 59.7%, 44.5%, and 37.3%, respectively, in the younger group, and 67.9%, 55.3%, and 53.8%, respectively, in the aged group. The 1-, 3-, and 5-year overall survival rates were 68.4%, 45.5%, and 38.9%, respectively, in the younger group, and 76.1%, 59.7%, and 53.9%, respectively, in the aged group. Alpha-fetoprotein ≥400 ng/mL, microvascular invasion, and tumor size >5 cm were independent risk factors for prognosis in both groups.
Conclusion
Younger patients in our center tended to present with more aggressive tumors and have a higher risk of recurrence. Our single-center experience suggests that younger patients should be assessed more rigorously before LT, while aged patients should be actively considered for LT after appropriate selection.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide.Citation1 It is estimated that there were 782,000 liver cancer cases and 746,000 liver cancer-related deaths worldwide in 2012.Citation2 Despite various therapeutic options such as liver resection, radiofrequency ablation, and transcatheter hepatic arterial chemoembolization, the prognosis remains generally poor, leading to 500,000 deaths per year.Citation3 The incidence of HCC is increasing rapidly worldwide.Citation4,Citation5
Liver transplantation (LT) remains the most effective treatment for small HCC with chronic liver disease and the best chance for end-stage liver disease.Citation6–Citation8 Sequential development from hepatitis B to liver cirrhosis and HCC is a typical phenomenon in most Chinese patients.Citation9 Fortunately, patients undergoing LT for hepatitis B virus-related cirrhosis have excellent long-term outcomes, with 5-year survival rates of 80%.Citation10 Various studies confirm that some preoperative variables such as tumor size, number of nodules, vascular invasion, histopathologic grading, and pre-LT serum alpha-fetoprotein (AFP) level are correlated with survival after LT in patients with HCC.Citation11–Citation13
Age is a complex prognostic factor in HCC and may play a paradoxical role in the prognosis of HCC patients.Citation14 Chen et alCitation15 found that male patients below 40 years of age with positive Hepatitis B surface antigen (HBsAg) had the worst survival in the early years because of increased AFP level and poor hepatic function. However, Zhang and SunCitation16 demonstrated that younger patients with liver cancer have higher liver cancer-specific survival after liver resection, despite the poorer biological behavior of this carcinoma.Citation16 The prognostic role of aging on survival following LT remains controversial.Citation17–Citation20
In this study, we evaluated the prognostic role of aging on survival after LT. In addition, we investigated the differences in clinical characteristics between aged and younger patients to explore the specific factors affecting the long-term prognosis between these two groups.
Materials and methods
Patients
A retrospective review of a prospectively maintained database in our institution (Affiliated First People’s Hospital, Shanghai Jiao Tong University, Shanghai, People’s Republic of China) was conducted. Between January 2001 and December 2011, a total of 314 HCC patients underwent LT; of these, 290 (259 males and 31 females) were included in this study. The median age of patients in our sample was 50 years (range 18–73 years). The mean follow-up time was 36.14 months (range 0.03–146.60 months). A total of 38 HCC patients with portal lymph node metastasis were included in the study. Among these patients, 31 were diagnosed on the basis of pathological specimens after surgery and it was not identified in preoperative evaluation (computed tomography [CT] and positron emission tomography-CT). The other seven of these patients were diagnosed as HCC with portal lymph node metastasis during recipient hepatectomy or by preoperative evaluation (CT and positron emission tomography-CT) and transplantation was performed because these patients met the Milan criteria. All diagnoses were confirmed by histopathologic examination. We excluded patients who had undergone LT more than once, had living donors or split-liver donors, were diagnosed with other malignancies in addition to HCC, or had missing pathology data.
Data collection
Patient baseline and clinical data, including age, sex, blood type, liver cirrhosis, hepatitis B virus status, hepatitis C virus status, pre-orthotopic liver transplantation (OLT) serum AFP level (stratification according to previous researchCitation21), Child–Pugh status,Citation22 tumor size, multinodular, microvascular invasion, portal lymph node metastasis, histologic grade (differentiated [well differentiated + moderately differentiated] and poorly differentiated), Milan criteria,Citation23 HCC recurrence, overall survival (OS), and recurrence-free survival (RFS), were recorded. Patients who were still alive or died from other causes (not HCC) were censored at their date of last visit or date of death. According to a previous study,Citation19 all recipients were divided into two groups: younger group (<49 years) and aged group (≥50 years).
This study was approved by the Institutional Review Board for Liver Transplantation Surgery, Shanghai First People’s Hospital, Shanghai, People’s Republic of China, under the guidelines of the Ethics Committee of the hospital and in accordance with the Declaration of Helsinki.Citation24 Due to the retrospective nature of the study, informed consent was waived.
Statistical analysis
All statistical analyses were performed using SPSS Version 19.0 statistical software (IBM Corporation, Armonk, NY, USA). Continuous data were expressed as the mean ± standard deviation or median (range), and discrete variables as frequencies. Categorical variables were compared using the Pearson’s χ2 test or Fisher’s exact test, whereas continuous variables were calculated with Student’s t-test. RFS and OS were assessed by Kaplan–Meier analysis and compared by the log-rank test. We also performed age-stratified survival analysis. Multivariate Cox proportional hazard regression was used to obtain hazard ratios and 95% confidence intervals associated with RFS and OS. The final models were determined by placing all variables with P<0.05 from the univariate analysis into a multivariate Cox regression model and using a forward stepwise variable selection process. Statistical significance was established at the P<0.05 level.
Results
Clinical demographics and follow-up data
The main demographic and clinical data of the 290 patients are listed in . The aged group had a significantly higher percentage of liver cirrhosis (P=0.048). Interestingly, there were more patients with AFP ≥400 ng/mL in the younger group (40.0% vs 26.5%, P=0.014). Moreover, the percentages of patients with tumor size >5 cm (P=0.038), poor differentiation (P=0.025), and portal lymph node metastasis (P=0.001) were all higher in the younger group. The mean follow-up time was 36.14 months (range 0.03–146.60 months). A total of 147 (50.7%) patients died during follow-up. The proportions of HCC recurrence and death were higher in the younger group (48.1% vs 36.1% and 55.6% vs 43.9%, respectively).
Table 1 Patient characteristics by age group
Kaplan–Meier survival analysis
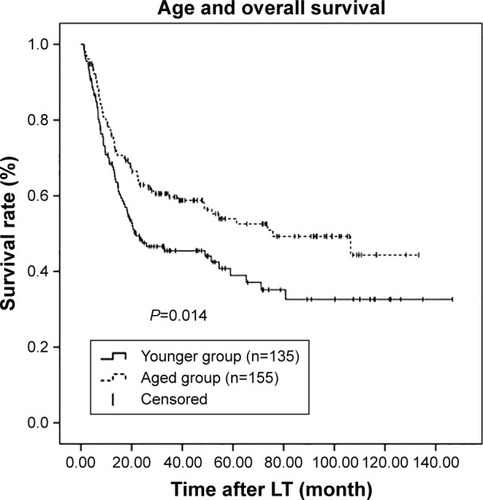
Kaplan–Meier survival curves were generated in combination with log-rank tests to compare RFS and OS across the age groups. There were significant differences in RFS and OS between these two groups (P=0.020 and P=0.014, respectively; and ). RFS and OS were remarkably higher in the aged group. The 1-, 3-, and 5-year RFS rates were 59.7%, 44.5%, and 37.3%, respectively, in the younger group and 67.9%, 55.3%, and 53.8%, respectively, in the aged group. Similarly, the 1-, 3-, and 5-year OS rates were 68.4%, 45.5%, and 38.9%, respectively, in the younger group and 76.1%, 59.7%, and 53.9%, respectively, in the aged group.
Figure 1 Kaplan–Meier survival estimates of RFS between the younger and aged groups.
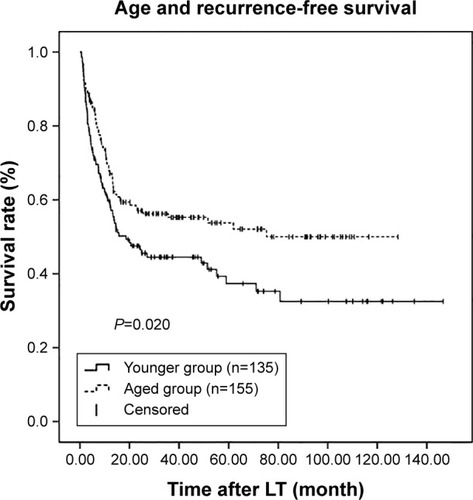
Figure 2 Kaplan–Meier survival estimates of OS between the younger and aged groups.
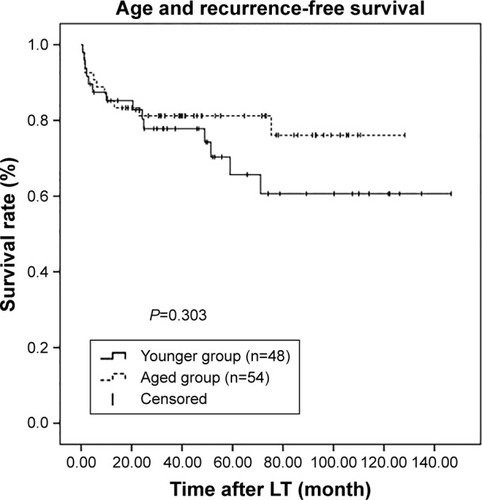
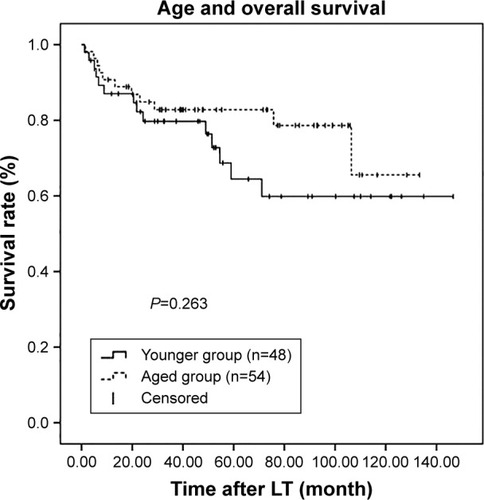
To investigate further, patients were assessed according to the Milan criteria. Among the 290 patients, 102 (35.2%) met these criteria, with 48 (47.1%) in the younger group and 54 (52.9%) in the aged group. However, among these selected patients, there were no statistically significant differences in both RFS and OS between two groups (P=0.303 and P=0.263, respectively; and ).
Multivariate Cox regression analysis of factors associated with RFS and OS stratified by age
Univariate and multivariate models were used to identify factors associated with RFS and OS for younger and aged HCC patients. All variables with P<0.05 in the univariate analysis (data no shown) were included in the multivariate Cox regression model. To avoid collinearity, the forward stepwise variable selection process was performed.
For RFS of the younger group, AFP ≥400 ng/mL, microvascular invasion, and tumor size >5 cm were independent risk factors, while microvascular invasion and tumor size >5 cm were independent risk factors for OS (). For both RFS and OS of the aged group, AFP ≥400 ng/mL, microvascular invasion, and tumor size >5 cm were independent risk factors ().
Table 2 Multivariate analysis of factors associated with RFS and OS in younger group
Table 3 Multivariate analysis of factors associated with RFS and OS in aged group
Discussion
The correlation between recipient age and clinical prognosis of HCC patients after LT remains controversial. In this single-center study, we evaluated the outcomes of patients in different age groups. Interestingly, our results demonstrated that the aged group was associated with better RFS and OS.
Recipient age as a prognostic factor for survival after LT has been studied for many years. Some studies indicated positive relationships,Citation20,Citation25,Citation26 while others yielded contradictory findings.Citation19,Citation27 These discrepancies may be due to the different age cut-off values and inclusion criteria applied in these studies as well as center-specific disparities in patient selection, operative techniques, and postoperative management.
A recent study conducted by Kim et alCitation19 with data obtained from the United Network for Organ Sharing Registry (10,238 patients) demonstrated that age in appropriately selected patients did not predict the success of LT in providing a long-term durable cure for HCC, and there were no significant differences in outcomes between patients aged 35–49 years and those aged 50–64 years. Similar patient selection criteria and age cut-off values were used in the present study, with six (2%) patients who were 65 years or older; however, our results led to a different conclusion. These conflicting outcomes arose from the fact that all HCC patients included in the study reported by Kim et al met the Milan criteria.Citation23 However, when we analyzed the data of selected patients who met the Milan criteria, we found no significant difference in the outcomes of the two groups, which is consistent with the results of the study reported by Kim et al. Nevertheless, our results were consistent with those reported by Wai et alCitation18 from a study of HCC patients aged less than 50 years, which was the same cut-off age applied in the present study. According to their experience, younger age (<50 years) was related to high risk of recurrence (62% vs 9%, P<0.001), and was an independent factor associated with poor RFS.
Aged patients had better prognosis in this study. One explanation for this may be that the younger patients tend to have more advanced disease, as indicated by the greater proportion with AFP ≥400 ng/mL, tumor size >5 cm, poor differentiation, and portal lymph node metastasis. This speculation has been confirmed in previous studies.Citation14,Citation16 Additionally, another interesting observation from our study is that there were more noncirrhotic HCC patients in the younger group. Beard et alCitation28 found higher risk pathologic features and an increased incidence of recurrence among noncirrhotic patients compared with cirrhotic patients. Similarly, younger age was associated with higher risk of recurrence in our study (48.1% vs 36.1%). In Asian countries, religion, culture, politics, and economy have a huge impact on organ allocation so that various patients with unresectable HCC which exceeded the Milan criteria are treated with LT, especially younger patients.Citation29 Consequently, there is a greater proportion of more severe HCC patients in the younger age group. Another explanation for the more favorable outcomes observed in the aged group lies in the more rigorous assessment of factors such as liver functional reserve and tolerance to surgery in older patients compared to younger patients.
However, age was not identified as an independent risk factor in the multivariate analysis conducted in our study (data not shown). Although Kim et alCitation19 presented positive results from their multivariate analysis, some crucial data such as AFP level and tumor features were not included in the Cox regression model. Nevertheless, Wai et alCitation18 demonstrated that younger age <50 years was an independent risk factor in a cohort of 77 HCC patients, with 86% exceeding the University of California, San Francisco criteria.Citation23 Compared with their study, we had a larger sample size, and our sample was a consecutive cohort. We conclude that age itself is not an independent risk factor but that the influence of age is associated with liver cirrhosis, AFP level, tumor size, histologic grade, and portal lymph node metastasis. In multivariate analysis stratified by age, both the younger and aged groups shared similar independent risk factors for RFS and OS: AFP level ≥400 ng/mL, microvascular invasion, and tumor size >5 cm, which is consistent with previous studies.Citation11,Citation18,Citation30
Inevitably, this analysis, similar to other studies, is limited because the patients in the two groups are not totally comparable in that preoperative assessment is likely to be more rigorous in older patients. An additional limitation is the extended period over which patients were selected for inclusion in the study. With advances in surgical techniques, improved management after surgery, and better immunosuppressive therapy, the clinical outcomes were improved over the period selection, resulting in heterogeneity among these patients.
In conclusion, our study showed that age ≥50 years was associated with longer RFS and OS compared with younger patients, but was not an independent risk factor. Younger patients in our center tended to present with more aggressive tumors and have a higher risk of recurrence. Our single-center experience suggests that younger patients should be assessed more rigorously before LT, while aged patients should be actively considered for LT after appropriate selection.
Disclosure
The authors report no conflicts of interest in this work.
References
- ParkinDMBrayFFerlayJPisaniPGlobal cancer statistics, 2002CA Cancer J Clin20055527410815761078
- SiegelRMaJZouZJemalACancer statistics, 2014CA Cancer J Clin201464192924399786
- MaluccioMCoveyARecent progress in understanding, diagnosing, and treating hepatocellular carcinomaCA Cancer J Clin201262639439923070690
- El-SeragHBHepatocellular carcinoma: recent trends in the United StatesGastroenterology20041275 Suppl 1S27S3415508094
- BruixJShermanMPractice Guidelines Committee AAftSoLDManagement of hepatocellular carcinomaHepatology20054251208123616250051
- LeeKKKimDGMoonISLeeMDParkJHLiver transplantation versus liver resection for the treatment of hepatocellular carcinomaJ Surg Oncol20101011475319798686
- BefelerASHayashiPHDi BisceglieAMLiver transplantation for hepatocellular carcinomaGastroenterology200512861752176415887162
- LlovetJMBurroughsABruixJHepatocellular carcinomaLancet200336293991907191714667750
- El-SeragHBRudolphKLHepatocellular carcinoma: epidemiology and molecular carcinogenesisGastroenterology200713272557257617570226
- CrespoGMarinoZNavasaMFornsXViral hepatitis in liver transplantationGastroenterology201214261373138322537446
- HanKTzimasGNBarkunJSPreoperative alpha-fetoprotein slope is predictive of hepatocellular carcinoma recurrence after liver transplantationCan J Gastroenterol2007211394517225881
- JonasSBechsteinWOSteinmullerTVascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosisHepatology20013351080108611343235
- YaoFYSaabSBassNMPrediction of survival after liver retransplantation for late graft failure based on preoperative prognostic scoresHepatology200439123023814752842
- ChenCHChangTTChengKSDo young hepatocellular carcinoma patients have worse prognosis? The paradox of age as a prognostic factor in the survival of hepatocellular carcinoma patientsLiver Int200626776677316911457
- ChenCHSuWWYangSSLong-term trends and geographic variations in the survival of patients with hepatocellular carcinoma: analysis of 11,312 patients in TaiwanJ Gastroenterol Hepatol200621101561156616928217
- ZhangWSunBImpact of age on the survival of patients with liver cancer: an analysis of 27,255 patients in the SEER databaseOncotarget20156263364125575810
- SharptonSRFengSHameedBYaoFLaiJCCombined effects of recipient age and model for end-stage liver disease score on liver transplantation outcomesTransplantation201498555756224717221
- WaiCTWoonWATanYMLeeKHTanKCYounger age and presence of macrovascular invasion were independent significant factors associated with poor disease-free survival in hepatocellular carcinoma patients undergoing living donor liver transplantationTransplant Proc201244251651922410059
- KimJKoMENelsonRAIncreasing age and survival after orthotopic liver transplantation for patients with hepatocellular cancerJ Am Coll Surg2014218343143824559955
- IshigamiMOnishiYKameiHImpact of recipient age and preoperative fasting blood glucose level as the risk factors of living donor liver transplantation in cirrhotic patients in the recent comprehensive era with knowledge of indications: recent status in a Japanese single centerHepatol Res201343111148115523413786
- ZhengSSXuXWuJLiver transplantation for hepatocellular carcinoma: Hangzhou experiencesTransplantation200885121726173218580463
- PughRNMurray-LyonIMDawsonJLPietroniMCWilliamsRTransection of the oesophagus for bleeding oesophageal varicesBr J Surg19736086466494541913
- MazzaferroVChunYSPoonRTLiver transplantation for hepatocellular carcinomaAnn Surg Oncol20081541001100718236119
- No authors listedWorld Medical Association declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjectsJAMA1997277119259269062334
- AndreouAGulSPascherAPatient and tumour biology predict survival beyond the Milan criteria in liver transplantation for hepatocellular carcinomaHPB (Oxford)201517216817525263399
- LevyMFSomasundarPSJenningsLWThe elderly liver transplant recipient: a call for cautionAnn Surg2001233110711311141232
- CrossTJAntoniadesCGMuiesanPLiver transplantation in patients over 60 and 65 years: an evaluation of long-term outcomes and survivalLiver Transpl200713101382138817902123
- BeardREHantoDWGautamSMiksadRAA comparison of surgical outcomes for noncirrhotic and cirrhotic hepatocellular carcinoma patients in a Western institutionSurgery2013154354555523777589
- ConcejeroAMChenCLEthical perspectives on living donor organ transplantation in AsiaLiver Transpl200915121658166119938130
- NagaiSYoshidaAFacciutoMIschemia time impacts recurrence of hepatocellular carcinoma after liver transplantationHepatology201561389590425099130