Abstract
Bosutinib is one of five tyrosine kinase inhibitors commercially available in the United States for the treatment of chronic myeloid leukemia. This review of bosutinib summarizes the mode of action, pharmacokinetics, efficacy and safety data, as well as the patient-focused perspective through quality-of-life data. Bosutinib has shown considerable and sustained efficacy in chronic myeloid leukemia, especially in the chronic phase, with resistance or intolerance to prior tyrosine kinase inhibitors. Bosutinib has distinct but manageable adverse events. In the absence of T315I and V299L mutations, there are no absolute contraindications for the use of bosutinib in this patient population.
Introduction
Chronic myeloid leukemia (CML) is a clonal myeloproliferative stem cell disorder characterized by the presence of a signature hybrid oncogene, the BCR–ABL.Citation1 The Philadelphia chromosome (Ph+) results from a reciprocal translocation between chromosome 9 and chromosome 22 that juxtaposes the two genes BCR and ABL and drives the leukemogenesis in CML.Citation2 The ABL gene encodes for a nonreceptor tyrosine kinase that becomes deregulated and constitutively active after the juxtaposition of BCR. BCR–ABL is central in controlling downstream pathways involved in cell proliferation, regulation of cellular adhesion, and apoptosis.Citation2 The understanding of the importance of this kinase activity in the pathophysiology of CML led to the development of tyrosine kinase inhibitors (TKI) that specifically target BCR–ABL. These agents became the mainstay of modern therapy in CML.Citation3
CML has a triphasic clinical course, and the majority of patients (~80%) are diagnosed during the early phase or the chronic phase (CP). However, and without effective treatment, CML invariably progresses to the advanced phases of the disease –the accelerated phase (AP) and the blast phase (BP). BP CML is a lethal refractory secondary leukemia with a short predicted survival.Citation2 CML is a relatively rare disorder occurring in 1.5 per 100,000;Citation4 however, and given the dramatic progress the therapeutic arena has witnessed in recent years, the prevalence of CML is increasing, and it is projected to increase further in the coming years.Citation5 Indeed, the number of people living with CML has doubled since 2001,Citation6 and with an estimated survival rate of 90% at 5 years and an annual mortality rate of 2%, the prevalence of CML in 20 years is projected to be 150,000 to 250,000 cases in the United States.Citation5
The goal of therapy in CML is the eradication of BCR–ABL. Eradication of BCR–ABL is associated with improvements in survival and a reduction in the risks of transformation to the advanced phases of the disease.Citation7,Citation8 This goal was not achieved with busulfan or hydroxyurea, but it was historically first achieved in the absence of allogeneic stem cell transplantation (alloSCT), with interferon alpha (IFN-α).Citation8,Citation9 Indeed survival of patients who achieve a complete cytogenetic remission with IFN-α is excellent and is close to 70% at 10 years. Unfortunately the tolerability of IFN-based therapies and the low response rates (less than 30%) have limited the broad applicability of this treatment.Citation10 Although alloSCT is a curative treatment, identification of a suitable donor, the age and comorbidities of the recipient, as well as the morbidity and mortality associated with this procedure, are the major limiting factors. The safety and tolerability, as well as the high response rates associated with TKI therapy, offered CML patients a new alternative that revolutionized their outcomes.Citation5,Citation11
Presently, five TKIs are commercially available in the United States, four of which are also available in many other countries, for the treatment of CML: imatinib (IM), Gleevec,® Novartis Pharmaceuticals Corporation; dasatinib (DAS), Sprycel,® Bristol-Myers Squibb Company, Princeton, NJ, USA; nilotinib (NIL), Tasigna,® Novartis Pharmaceuticals Corporation; bosutinib (BOS), Bosulif,® Pfizer, Inc, New York, NY, USA; and ponatinib (PON), Iclusig,® Ariad Pharmaceuticals, Cambridge, MA, USA.
IM was the first approved TKI by the US Food and Drug Administration (FDA) for CML and other Ph+ leukemias in 2001.Citation12 DAS and NIL were approved in 2006 and 2007, respectively, for IM-resistance or intoleranceCitation13,Citation14 and later approved as front-line therapy in 2010. In 2012, both BOS and PON were granted FDA approval in the US for resistant CML.Citation15,Citation16 BOS is a SRC–ABL inhibitor approved in the US for the treatment of chronic, accelerated or blast phase Ph+ CML in adult patients with resistance or intolerance to prior therapy. This review will focus on pharmacology, mode of action, pharmacokinetics, safety, and efficacy of BOS for the treatment of CML.
Review of pharmacology, mode of action, pharmacokinetics of BOS
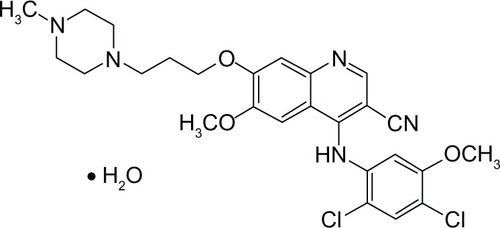
BOS is a 4-anilino-3-quinolinecarbonitrile. The molecular weight is 548.46 grams per mole (monohydrate); 530.46 grams per mole (anhydrous). The molecular formula is C26H29Cl2N5O3 × H2O; and the chemical formula is 3-Quinolinecarbonitrile, 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-, hydrate (1:1) 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-3-quinolinecarbonitrile monohydrate 4-[(2,4-Dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methylpiperazin-1-yl) propoxy]quinoline-3-carbonitrile monohydrate. The molecular structure of BOS is shown in .Citation16
Figure 1 Molecular structure of bosutinib.Citation16
Mode of action
BOS inhibits BCR–ABL kinase, as well as the SRC-family kinases including SRC, Lyn, and Hck. In mice, treatment with BOS inhibited growth of IM-resistant myeloid tumors.Citation17,Citation18
Pharmacokinetics
Following a single oral dose of BOS 500 mg with food, median time-to-peak concentration (Tmax) was 4–6 hours. BOS exhibited linear kinetics with dose proportional increases in area under the curve (AUC) and a maximum concentration (Cmax) 200 and up to 800 mg. After 15 daily doses of BOS 500 mg, Cmax was 200 (±12) ng/mL, and the AUC was 3650 (±425) ng/mL. The volume of distribution was 6080 (±1230) L. BOS binds in a nonconcentration-dependent pattern to plasma proteins, both in vitro (94%) and in vivo (96%).Citation19 The mean terminal phase elimination half-life (t1/2) was 22.5 (±1.7) hours, and the mean clearance is 189 (±48) L/h. BOS is primarily metabolized by cytochrome P450 (CYP)-3A4, and inactive plasma metabolites include the oxydechlorinated BOS (19% of parent exposure) and N-desmethylated BOS (25% of parent exposure). BOS N-oxide is a minor circulating metabolite.Citation16 BOS is excreted in feces with only 1% excreted in the urine.Citation19
In a dedicated hepatic impairment trial, a single dose of BOS 200 mg was administered with food to hepatically impaired volunteers (Child–Pugh classes A, B, and C), and showed increased Cmax by 1.5- to 2.4-fold, and a 1.9- to 2.3-fold increased AUC.Citation16 CYP3A inhibitors, such as ketoconazole, increase BOS Cmax and AUC 5.2-fold and 8.6-fold, respectively.Citation16 CYP3A inducers, such as rifampicin, decrease BOS Cmax and AUC by 86% and 94%, respectively. When given with pH-altering medications, such as lansoprazole, Cmax and AUC decrease by 46% and 26%, respectively.Citation16
Clinical trials addressing efficacy of BOS
BOS in previously treated CML
In a Phase 1–2 study, BOS was initially administered to patients with IM-resistant or IM-intolerant CML to determine the Phase 2-recommended dose (P2RD) (Phase 1) and to evaluate the safety and efficacy of this dose (Phase 2). In the dose-escalation part of the study, BOS at 500 mg once daily dosing was considered the P2RD, as skin toxicity was the limiting dose at 600 mg per day. The study enrolled 288 patients with IM-resistant (200) or IM-intolerant (88) CML, and as NIL and DAS became commercially available, the eligibility criteria were modified to include patients previously treated with these agents. At 24 weeks, 86% of CP IM-resistant and 85% IM-intolerant CML patients achieved CHR, which was sustained in 72% and 87%, respectively. Major cytogenetic remission (MCyR, defined as a decrease in Ph+ metaphases to ≤35%) was achieved in 54% of CP IM-resistant and 49% of IM-intolerant patients and maintained in 72% CP IM-resistant and 92% of IM-intolerant patients, of which 41% each had a complete cytogenetic response (CCyR)Citation20 (). The overall survival (OS) at 2 years was 88% and 98%, with a progression-free survival (PFS) of 72% and 89% for CP IM-resistant and IM-intolerant patients, respectively. BOS was also effective when administered as a third-line agent to 119 CP CML patients with resistance or intolerance to IM, DAS, or NIL,Citation21 of which 64%–81% achieved CHR, 32%–51% MCyR that was complete in 23%–41%. The highest response rates were observed in the IM- and DAS-intolerant, while IM- and DAS-resistant patients had the lowest responses. PFS was 75%, and OS was 84% at 2 years ().
Table 1 Efficacy of bosutinib as a second or third-line treatment for CP CML
BOS for newly diagnosed CML
A Phase 3 trial compared head-to-head BOS to IM in newly diagnosed CP–CML (in the Bosutinib Efficacy and Safety in Newly Diagnosed Chronic Myeloid Leukemia [BELA] trial).Citation22 A total of 502 patients was randomly assigned 1:1 to BOS 500 mg per day or IM 400 mg per day. The study did not achieve the primary end point, given that the CCyR rate at 12 months was not different in BOS (70%; 95% confidence interval [CI]; 64%–76%) versus IM-treated patients (68%; 95% CI; 62%–74%). The major molecular response (MMR) rate at 12 months was higher with BOS (41%; 95% CI; 35%–47%) as compared with IM (27%; 95% CI; 22%–33%), and time to achieve CCyR and MMR was faster with BOS compared with IM (12.9 versus 24.6 weeks and 37.1 versus 72.3 weeks, respectively).
Transformations to AP or BP CML as well as CML-related deaths were comparable in both arms of the study (2% versus 4%). BOS was therefore not proposed for approval for the upfront treatment of the newly diagnosed CP CML patients.
BOS for advanced phases CML
The Phase 1–2 study included 134 patients with 63 AP, 48 BP CML, and 23 Ph+ acute lymphoblastic leukemia (ALL).Citation23 Median follow-up was 8.3 months. Prior therapy in addition to IM included IFN-α, DAS, NIL, and alloSCT. Overall hematological response (which included CHR, return to a second CP, and no evidence of leukemia) was achieved in 64% of AP, 32% of BP, and 25% of Ph+ ALL patients. CCyR was achieved in 33% of AP, 29% of BP, and 100% of the two evaluable Ph+ ALL patients. PFS was 11.6 months for AP CML versus 7.8 months and 2.7 months for BP CML and Ph+ ALL, respectively.
Safety and tolerability
BOS is overall well-tolerated and, similar to other TKIs, is associated with both specific and nonspecific side effects. Gastrointestinal adverse events (AEs) are common (81% diarrhea; 43% nausea; 32% vomiting), and specific side effects are associated with the use of BOS.Citation21 These gastrointestinal AEs are often Grades 1–2 (90%); have an early onset (median 1.5 days for diarrhea; nausea, 3.5 days; vomiting, 19.5 days, respectively); are usually of short duration (median duration, 2 days); and are self-limited. Diarrhea is effectively managed with antidiarrheal agents, such as loperamide or diphenoxylate/atropine. In the Phase 1–2 trial, dose interruptions and reductions occurred in 15% and 6%, respectively. Rechallenging after temporary interruption of BOS was successful; there was no recurrence of diarrhea in 95%. Diarrhea led to BOS discontinuation in 3%.Citation20 Elevation of alanine aminotransferase (ALT) and elevation of aspartate aminotransferase (AST) occurs in 10%–13% and is Grades 1–2 in 90%, Grade 3 and Grade 4 in 10%.Citation21 These ALT and AST elevations tend to occur in the first month after initiation of BOS (median 21.5 and 29 days, respectively),Citation21 are relatively short-lived (median 15 and 9 days, respectively), and have led to dose reductions, interruptions, or discontinuation (1%–3%) in the Phase 1–2 trials. However, among patients who required temporary dose interruption for ALT and/or AST elevations and were subsequently rechallenged with BOS, 90% either did not have a recurrence of ALT/AST events or had a recurrence but did not require permanent discontinuation of BOS.
Skin rash occurred in 22% of patients and resolved with time and required topical supportive care.Citation21 Overall, 10% of patients experienced Grade 3–4 skin toxicity. Skin manifestations include: macular, papular, and pruritic rash, acne, allergic dermatitis, folliculitis, and skin exfoliation.Citation20
Similar to what is observed with other TKIs, myelosuppression presenting as pancytopenia or single lineage cytopenia is common in BOS-treated patients. Of note, the patient population enrolled in the Phase 1–2 trial had baseline Grade 3–4 hematologic laboratory abnormalities prior to initiation of BOS (thrombocytopenia 31%; neutropenia, 25%; anemia 16%), which affects the interpretation of the reported incidence from this trial. Thrombocytopenia represented the most common Grade 3–4 AE and Grade 3–4 laboratory abnormality (24%).Citation20 The median time to myelosuppression was 22 days, and median duration was 14 days. Myelosuppression was managed primarily with dose modifications, with 48% undergoing temporary interruption and 33% having dose reduction. Myelosuppression led to discontinuation of treatment in 6%.
Fluid retention is rare, but it has been reported in BOS-treated patients manifesting with pleural effusion (8%, Grade 3–4 in 2%), and less commonly pericardial effusion, pulmonary edema, or peripheral edema. Pleural effusion has late onset (median 468 days), and a median duration of 19 days. In the Phase 1–2 trial, pleural effusion led to dose interruptions in 43% and to dose reductions in 17%. Only 2 (<1%) patients discontinued BOS due to pleural effusions.Citation21 Among patients who experienced pleural effusion during BOS treatment, 38% had a prior history of pleural effusion and 30% attributed prior TKI discontinuation to pleural effusion (85% had effusions with DAS).
Other nonspecific AEs associated with BOS treatment include fatigue (24%), cardiac events (14%), muscle spasms (3%), musculoskeletal pain (3%), creatine kinase elevations (2%), and myalgias (5%).Citation20
Risk factors for development of AE
Contrary to the gastrointestinal AEs, the hematologic AEs associated with BOS treatment are more common among advanced phase patients (57% versus 34%). Additionally, in the Phase 1–2 trial, dose modifications due to hematological AEs were more common in the IM-intolerant (83%) patients as compared to IM-resistant patients (65%), leading to more drug interruptions (57% versus 44%).Citation24 Compared with younger patients, vomiting, constitutional symptoms, cardiac events, pleural effusions, and dyspnea are reported more frequently among the older patients. In contrast, liver function test elevation and abdominal pain were more common in younger patients. Grade 3–4 hematologic abnormalities were generally similar between age groups. Older patients were more likely to undergo treatment modifications or to discontinue BOS due to an AE.
Activity of BOS in the presence of ABL domain mutation
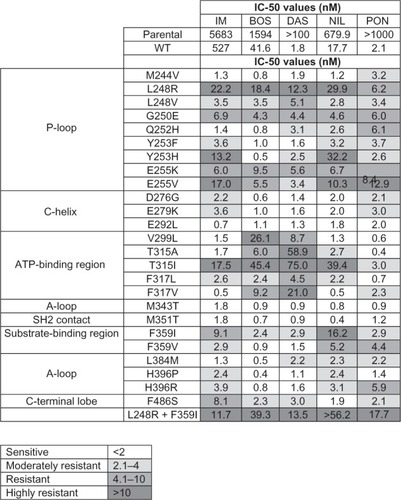
ABL kinase domain mutation can be crucial in directing therapy in the CML patient with resistance to first or second generation TKIs. In the Phase 1–2 trial, clinical activity of BOS was observed across baseline ABL kinase domain mutations except when T315I and V299L were detected. BOS is active despite the presence of mutations conferring resistance to DAS (F317L) or NIL (Y253F/H, E255K/V, and F359C/I/SV)().Citation25,Citation26 The most common emerging mutations during BOS therapy are T315I and V299L, and all patients who developed these mutations had progressive disease.Citation25 Overall, CHR and MCyR rates are similar among CP patients with and without detectable mutations; however, the presence of a mutation negatively impacts response rates in advanced phases of CML and Ph+ ALL (CHR, 17% versus 39%; MCyR, 24% versus 37%).Citation25
Figure 2 Comparison of in vitro sensitivity to IM, BOS, DAS, NIL, and PON of wild type and mutant BAF3 cell lines harboring clinically relevant ABL domain kinase mutations.
Abbreviations: IC, inhibitory concentration; nM, nanomolar; WT, wild type; IM, imatinib; BOS, bosutinib; DAS, dasatinib; NIL, nilotinib; PON, ponatinib.
Patient-focused perspectives
A health-related quality of life and the Functional Assessment of Cancer Therapy–Leukemia (FACT–Leu) evaluation were obtained prior to and throughout BOS treatment in the CP cohort of the Phase 1–2 trial.Citation27 Improvements on several FACT-Leu scales, including emotional well-being, overall FACT Leukemia Total and General scores were noted after the initiation of BOS. In particular, the patients’ Physical Well-being and Functional Well-Being subscales, which measure issues such as lack of energy, presence of pain, ability to work, and ability to enjoy life, showed little impairment during BOS treatment.Citation27
Conclusion
BOS is an effective agent in the armamentarium against CML. In the absence of a specific ABL kinase domain mutation, health care providers have the luxury of selecting from an impressive portfolio of active drugs. Factors playing an important role in these therapeutic decisions include age, comorbidities, dosing schedule, patient and family member preference, and last but not least, the physician’s preference. BOS has shown considerable and sustained efficacy in patients with resistant or intolerant Ph+ leukemia, and has distinct but manageable adverse events. In the absence of T315I and V299L mutations, there are no absolute contraindications for the use of BOS in Ph+ leukemias.
Disclosure
The authors report no conflicts of interest in this work.
References
- FaderlSTalpazMEstrovZO’BrienSKurzrockRKantarjianHMThe biology of chronic myeloid leukemiaN Engl J Med1999341316417210403855
- SawyersCLChronic myeloid leukemiaN Engl J Med1999340171330134010219069
- KantarjianHMTalpazMGilesFO’BrienSCortesJNew insights into the pathophysiology of chronic myeloid leukemia and imatinib resistanceAnn Intern Med20061451291392317179059
- JemalASiegelRWardECancer statistics, 2006CA Cancer J Clin200656210613016514137
- HuangXCortesJKantarjianHEstimations of the increasing prevalence and plateau prevalence of chronic myeloid leukemia in the era of tyrosine kinase inhibitor therapyCancer2012118123123312722294282
- SEER Cancer Statistics Review, 1975–2006, [webpage on the Internet]Bethesda, MDNational Cancer Institute2008 [cited 04/16/2012]. Available from: http://seer.cancer.gov/csr/1975_2006/Accessed 06/11/2013
- HughesTPHochhausABranfordSLong-term prognostic significance of early molecular response to imatinib in newly diagnosed chronic myeloid leukemia: an analysis from the International Randomized Study of Interferon and STI571 (IRIS)Blood2010116193758376520679528
- KlokeOOpalkaBNiederleNInterferon alfa as primary treatment of chronic myeloid leukemia: long-term follow-up of 71 patients observed in a single centerLeukemia200014338939210720131
- BonifaziFde VivoARostiGChronic myeloid leukemia and interferon-alpha: a study of complete cytogenetic respondersBlood200198103074308111698293
- O’BrienSGGuilhotFLarsonRAImatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemiaN Engl J Med200334811994100412637609
- BjörkholmMOhmLElorantaSSuccess story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008J Clin Oncol201129182514252021576640
- US Food and Drug AdministrationImatinib Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021588s024lbl.pdfAccessed March 19, 2013
- US Food and Drug AdministrationFDA grants accelerated approval of a new dosing regimen of dasatinib (Sprycel) [press release]Silver Springm, MDUS Food and Drug Administration2007 [November 13]. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm129236.htmAccessed March 19, 2013
- US Food and Drug AdministrationNilotinib (Tasigna) Available at: [press release]Silver Spring, MDUS Food and Drug Administration2010 [June 18]. Available from: http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm216218.htmAccessed March 19, 2013
- US Food and Drug AdministrationPonatinib [press release]Silver Spring, MDUS Food and Drug Administration2012 [December 17]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm332368.htmAccessed March 23, 2013
- US Food and Drug AdministrationBosutinib tablets [press release]Silver Spring, MDUS Food and Drug Administration2012 [September 5]. Available from: http://www.fda.gov/Drugs/InformationOnDrugs/ApprovedDrugs/ucm318203.htmAccessed March 17, 2013
- PuttiniMColucciaAMBoschelliFIn vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cellsCancer Res20066623113141132217114238
- Remsing RixLLRixUColingeJGlobal target profile of the kinase inhibitor bosutinib in primary chronic myeloid leukemia cellsLeukemia200923347748519039322
- AbbasRHugBALeisterCGaaloulMEChalonSSonnichsenDA phase I ascending single-dose study of the safety, tolerability, and pharmacokinetics of bosutinib (SKI-606) in healthy adult subjectsCancer Chemother Pharmacol201269122122721691746
- CortesJEKantarjianHMBrümmendorfTHSafety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinibBlood2011118174567457621865346
- KhouryHJCortesJEKantarjianHMBosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failureBlood2012119153403341222371878
- CortesJEKimDWKantarjianHMBosutinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: results from the BELA trialJ Clin Oncol201230283486349222949154
- Gambacorti-PasseriniCKhouryHJSafety and efficacy of BOS in patients with AP and BP CML and Ph+ ALL following resistance/intolerance to IM and other TKIs: update from study SKI-200Presented at the American Society of Clinical OncologyJune 4–8, 2010Chicago, IL, USA Abstract 6509
- Gambacorti-PasseriniCBrummendorfTHCortesJEEvolution of bosutinib (BOS) toxicity in patients (pts) with Ph+ leukemia after resistance/intolerance to prior therapyPresented at the American Society of Clinical OncologyMay 31–June 4, 2013Chicago, IL, USA
- KhouryHJCortesJEGambacorti-PasseriniCActivity of bosutinib by baseline and emergent mutation status in Philadelphia chromosome-positive leukemia patients with resistance or intolerance to other tyrosine kinase inhibitorsPresented at the Annual Meeting of the American Society of HematologyDecember 11, 2011San Diego, CA, USA
- RedaelliSMologniLRostagnoRThree novel patient-derived BCR/ABL mutants show different sensitivity to second and third generation tyrosine kinase inhibitorsAm J Hematol201287E125E12823044928
- TraskPCCellaDBessonNKellyVMassziTKimDWHealth-related quality of life of bosutinib (SKI-606) in imatinib-resistant or imatinib-intolerant chronic phase chronic myeloid leukemiaLeuk Res201236443844222036634