Abstract
Subacute sclerosing panencephalitis is a progressive neurodegenerative disease. It usually occurs 7–10 years after measles infection. The clinical course is characterized by progressive cognitive decline and behavior changes followed by focal or generalized seizures as well as myoclonus, ataxia, visual disturbance, and later vegetative state, eventually leading to death. It is diagnosed on the basis of Dyken’s criteria. There is no known cure for subacute sclerosing panencephalitis to date, but it is preventable by ensuring that an effective vaccine program for measles is made compulsory for all children younger than 5 years in endemic countries.
Keywords:
Introduction
Subacute sclerosing panencephalitis, commonly known as SSPE, is a progressive neurodegenerative disease caused by the persistence of measles infection commonly seen in children and young adults.Citation1 SSPE was previously known as Dawson’s inclusion body encephalitis as Dawson in 1933 and 1934 reported cellular inclusions in the cerebral lesions of patients with SSPE, thus resulting in this disease being labeled as Dawson inclusion body encephalitis.Citation2 Ten years later, Brain et alCitation3 reported similar conditions with further case reports, and later, the term SSPE was coined. Electron microscopic evidence of paramyxovirus was established between 1967 and 1969.Citation4
Measles is a highly contagious RNA virus of the paramyxoviridae family and the genus morbillivirus. It is an airborne disease and transmitted via nasopharyngeal droplets. The virus is highly lymphotropic, affecting dendritic cells, alveolar macrophages, and subsets of B and T cells in the lymphoid tissue of the lower respiratory tract, and later, it infiltrates the epithelium of the upper respiratory tract. Acute complications include otitis media, pneumonia, diarrhea, and postinfectious encephalitis.Citation5 Neurological complications of measles involve post-measles encephalitis, measles inclusion body encephalitis, transverse myelitis, and SSPE.Citation6 The risk of serious complications and death is increased in children younger than 5 years and adults older than 20 years.Citation7 This disease is preventable, and immunization against measles via live attenuated vaccine has been available for more than 45 years.Citation8
SSPE usually occurs 7–10 years after measles infection, but the latency varies from 1 month to 27 years.Citation9 A shorter latency has been reported in intrafamilial cases of SSPE as well as in children who were affected at an earlier (<2 years) age such that the incidence was 18/100,000 in children younger than 5 years and 1.1/100,000 in those children with measles after 5 years.Citation10,Citation11 It is caused by the cerebral involvement of the measles virus, which causes destruction of the neurons. The pathogenesis of SSPE is yet to be elucidated, but it has been shown to be caused by the wild strains and not by the vaccine strains, which has been supported by genetic studies.Citation12 The strains of measles virus causing SSPE have multiple point mutations in their genomes, especially in the gene encoding for the matrix protein gene.Citation13 Studies have shown that the capacity of wild-type measles virus strains to cause SSPE results from their increased capacity to spread and that this is partially due to a tri-residue motif, P64, E89, and A209 (PEA), in their M proteins, which is absent in vaccine and lab-adapted strains.Citation14 Mutations in M proteins result in interference with the assembly of new viral particles and their budding, which form viral particles that are transmitted via ribonucleic protein with a trans-synaptic spread.Citation15 Immaturity of the cellular immunity mechanism has been critical as suggested by earlier age of acquiring measles infection resulting in higher incidences of SSPE.Citation11 Although other viruses have been studied in association with SSPE, there is no data to support their role in causation of the disease.Citation13
The worldwide prevalence of SSPE has declined to 1 per 100,000 cases of measles due to better immunization coverage in developed countries.Citation7 There is not only geographical variation in the prevalence of SSPE but economic development also contributes to the falling trends. Developed countries such as USA have reported an incidence of 6.5–11 cases per 100,000 acute measles infections.Citation12 European countries like Turkey have reported an incidence of 2.2 cases per million in their population.Citation16 Developing countries like Pakistan have reported an estimated incidence of 10 cases per million in their population.Citation17 The highest rate that has ever been reported is from Papua New Guinea, which is 51 cases per one million during 2007–2009.Citation18 Although there is no gender predisposition, but SSPE has been seen more commonly in boys.Citation19 The risk of SSPE is higher if the onset of measles is at a younger age, in low socioeconomic class, in cases with low parental education and large family size.Citation4
The clinical course is characterized by progressive cognitive decline and behavior changes followed by focal or generalized seizures as well as myoclonus, ataxia, visual disturbance, and later vegetative state.Citation20,Citation21 Patients suffering from SSPE die within few years of initial clinical presentation although there have been rare case reports of spontaneous remission.Citation20 Epilepsy has been reported in one-third of the patients with SSPE.Citation22
Jabbour et alCitation23 have divided the clinical manifestations into four stages. Stage I is characterized by irritability, dementia, social withdrawal, lethargy, and regression of speech; stage II is characterized by various types of movement disorders such as dyskinesia, dystonia, and myoclonus. Stage III is consistent with extrapyramidal symptoms, decerebrate posturing, and spasticity, while the stage IV is characterized by loss of function of cerebral cortex with signs of vegetative state, autonomic failure, and akinetic mutism. Atypical presentations have been described including isolated psychiatric manifestations, poorly controlled seizures, and isolated extrapyramidal symptoms, such as dystonia, chorea, hemi-parkinsonism, etc. Occasionally, a stroke-like onset has also been described. There may be a transient plateau period or slight improvement in some patients, but classically it has a relentless pattern associated with high mortality.Citation24 Differential diagnoses include epilepsy and psychiatric illnesses in early stages along with other viral encephalitides, atypical multiple sclerosis, leukodystrophies, variant Creutzfeldt–Jakob disease, and neurometabolic encephalopathies.Citation25 The diagnosis of SSPE is often considered late in developed countries owing to its rare occurrence and the nonspecific clinical manifestations at onset.Citation26
Visual loss as an initial presentation has also been described.Citation27 Ocular findings are seen in almost 50% of cases. A variety of neuroopthalmological and retinal findings are associated with SSPE, and the classic lesion is focal necrotizing macular retinitis. There may be retinal hemorrhages, edema, and detachment. Vitreal inflammation is not seen in SSPE. Optic disc changes include papillitis, papilledema, and disc pallor. Retinal involvement may settle with time or eventually lead to scarring. Ophthalmic symptoms may precede the neurological symptoms of SSPE. Other symptoms that may occur include cortical blindness, gaze palsies, ptosis, and nystagmus.Citation28
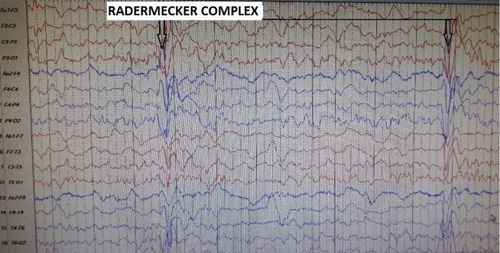
The diagnosis is based on the Dyken’s criteria, which include two major and four minor criteria. Major criteria include 1) raised anti-measles antibody titers in cerebrospinal fluid (CSF) greater than or equal to 1:4 or ratio greater than or equal to 1:256 in serum, and 2) typical or atypical clinical history (typical includes acute or rapidly progressive, subacute progressive, chronic progressive, and chronic relapsing–remitting, while atypical includes seizures, prolonged stage I, and unusual age of presentation that is either in infancy or adulthood). Minor criteria include the following: 1) characteristic electroencepahlograhic findings that include periodic, generalized, bilaterally synchronous and symmetrical high-amplitude slow waves that recur at regular intervals of 5–15 seconds called periodic slow-wave complexes also known as “Radermecker” complexes (). The interval between complexes is generally fixed, but variation in the interval between periodic discharges may also be seen, also known as pseudo-periodic or quasi-periodic discharges.Citation29,Citation30 2) CSF globulin levels greater than 20% of the total CSF protein. 3) Characteristic histopathological findings on brain biopsy including inflammatory changes in the meninges and cerebral parenchyma necrotizing leukoencephalitis with diffuse demyelination; viral inclusion bodies in neurons; oligodendrocytes and astrocytes; neuronal loss; and astrocytosis. 4) Specialized molecular diagnostic test to identify wild-type measles virus mutated genome. Usually two major criteria plus one minor criterion are required, but if the features are atypical, then histopathological or molecular evidence may be required.Citation29,Citation30 Interestingly, histopathological studies carried out after autopsy of individuals without SSPE showed approximately 20% having detectable measles virus in the brain.Citation31 However, just the presence of RNA without satisfaction of Dyken’s criteria would not mean that the person has SSPE.
Figure 1 EEG of a patient with SSPE.
Abbreviations: EEG, electroencephalogram; SSPE, subacute sclerosing panencephalitis.
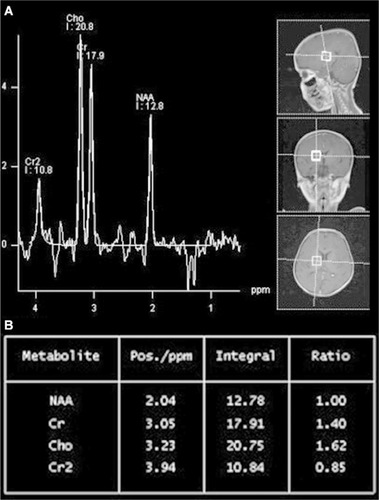
Neuroimaging may be helpful but is not characteristic of SSPE. During the early stages, magnetic resonance (MR) imaging of the brain may show decreased gray matter volume, especially within the frontotemporal cortex, amygdala, and cingulate gyrus. As the disease progresses, hyperintensities on T2-weighted images in the cerebral cortex, periventricu-lar white matter, basal ganglia, and brainstem may develop (). Eventually, the MR images will reveal diffuse cortical atrophy, as evidenced by enlarged sulci and ventriculomegaly. MR spectroscopy findings range from increased choline-to-creatinine ratio and inositol-to-creatinine ratios along with normal N-acetyl aspartate-to-creatinine ratios to decreased N-acetyl aspartate-to-choline and N-acetyl aspartate-to-creatinine ratio correlating to loss of brain volume ().Citation19,Citation32–Citation36
Figure 2 (A–C) The MRI of a 6-year-old girl with SSPE.
Abbreviations: MRI, magnetic resonance imaging; SSPE, subacute sclerosing panencephalitis.
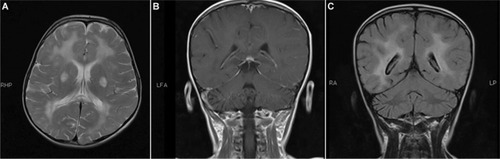
Figure 3 (A and B) The MRS of the 6-year-old girl with SSPE.
Abbreviations: MR, magnetic resonance; MRS, magnetic resonance spectroscopy; NAA, nacetyl aspartate; Cr, creatinine.
Currently, there is no cure for SSPE, and eradication by effective vaccination program is considered to be more beneficial and cost-effective than any other high-level forms of control.Citation6 Measles-containing vaccines are a part of the childhood vaccination schedule in all countries. Current World Health Organization (WHO) policy is that “Reaching all children with 2 doses of measles vaccine should be standard for all national immunization programs”.Citation37 Despite this, global coverage with the first dose of measles vaccine has largely stagnated since 2004. Six WHO regions have measles elimination goals for the year 2020, but the World Health Assembly has still not endorsed the eradication of this disease. In 2012, Measles and Rubella Initiative published a Global Measles and Rubella Strategic Plan 2012–2020, which aimed to achieve elimination of these two diseases in 5 WHO regions by 2020. By the end of 2015, none of these milestones had been met. Although the number of countries with measles vaccine 1 coverage of >90% has risen between 2010 and 2015, we are still a long way from a global measles eradication.Citation37
Supportive treatment including management of seizures and other complications is the mainstay.Citation11 Divalproate sodium is one of the common antiepileptics employed.Citation38 There are no standard treatment protocols for the treatment of SSPE. Antiviral drugs and immunomodulators are used in the treatment of SSPE. Even though there are many drugs that have been tried in the treatment of SSPE, inosine pranobex, interferon alfa, ribavirin, and lamivudine are the most commonly used drugs in routine clinical practice. These have been used either singly or in combinations. Inosine pranobex (Isoprinosine, Inosiplex) is an antiviral drug with immunomodulatory effects. It is a synthetic compound. It is given orally in doses of 100 mg/kg/day (with a maximum dose of 3,000 mg/day) in three divided doses to patients with SSPE. Abnormalities in serum and urinary uric acid and occasional nausea have been reported with Isoprinosine.Citation39 Interferon alfa is an immunomodulator drug. It is preferably given via the intraventricular route as it has very poor penetration of the blood–brain barrier. Ribavarin and lamivudine have also been tried with no great success.
Ketogenic diet has also been tried; it was found to temporarily reduce the myoclonic jerks.Citation40 Steroids and intravenous immunoglobulin are no longer recommended for the treatment of SSPE.
Future therapies are incorporating antiapoptotic agents, and RNAi is being experimented upon and may be beneficial in future.Citation41
The prognosis for SSPE remains guarded. Mortality has been reported in 95% of the patients.Citation42 The average life span of a patient suffering from SSPE is 3.8 years (45 days–12 years).Citation16 Another study has proposed that the mean survival in children is about 1 year 9 months to 3 years.Citation11
Conclusion
In short, SSPE is a potentially lethal disease and causes a huge burden both emotionally and financially, affecting not only the family but the country as whole. Furthermore, as this is seen more in the underdeveloped countries, the economic burden to these nations is huge. There is a strong need to improve the vaccination status of countries where the incidence of measles is high as it may be the only way to eradicate this devastating condition. This requires a global and political will and ownership by the individual countries.
Acknowledgments
The ethical review committee of the Aga Khan University agreed that no patient consent for the use of the figures was needed as patient data was kept anonymous.
Disclosure
The authors report no conflicts of interest in this work.
References
- IbrahimSHAmjadNSaleemAFChandPRafiqueAHumayunKNThe upsurge of SSPE—a reflection of national measles immunization status in PakistanJ Trop Pediatr201460650
- DawsonJRCellular inclusions in cerebral lesions of epidemic encephalitis: second reportArch Neurol Psych1934314685700
- BrainWRGreenfieldJRusselDSSubacute inclusion encephalitis (Dawson type)Brain194871436538518114337
- CampbellHAndrewsNBrownKMillerEReview of the effect of measles vaccination on the epidemiology of SSPEInt J Epidemiol20073661334134818037676
- HolzmannHHengelHTenbuschMDoerrHEradication of measles: remaining challengesMed Microbiol Immunol2016205320120826935826
- GargRKSubacute sclerosing panencephalitisJ Neurol2008255121861187118846316
- StrebelPMPapaniaMJDayanGHHalseyNAMeasles vaccineVaccines20085353398
- OrensteinWAStrebelPMPapaniaMSutterRWBelliniWJCochiSLMeasles eradication: is it in our future?Am J Public Health200090101521152511029981
- CruzadoDMasserey-SpicherVRouxLDelavelleJPicardFHaenggeliCAEarly onset and rapidly progressive subacute sclerosing panencephalitis after congenital measles infectionEur J Pediatr2002161843844112172828
- SharmaVGuptaVBEisenhutMFamilial subacute sclerosing panencephalitis associated with short latencyPediatr Neurol200838321521718279759
- GutierrezJIssacsonRSKoppelBSSubacute sclerosing panencephalitis: an updateDev Med Child Neurol2010521090190720561004
- BelliniWJRotaJSLoweLESubacute sclerosing panencephalitis: more cases of this fatal disease are prevented by measles immunization than was previously recognizedJ Infect Dis2005192101686169316235165
- AnlarBPinarAAnlarFYViral studies in the cerebrospinal fluid in subacute sclerosing panencephalitisJ Infect200244317618012099745
- KwederHAinouzeMBrunelJGerlierDManetEBucklandRMeasles virus: identification in the M protein primary sequence of a potential molecular marker for subacute sclerosing panencephalitisAdv Virol2015201576983726587021
- ReissCSNeurotropic Viral Infections2nd edReissCSSwitzerlandSpringer International Publishing2016370
- GulerSKucukkocMIscanAPrognosis and demographic characteristics of SSPE patients in Istanbul, TurkeyBrain Dev201537661261725270981
- TakasuTKondoKAhmedASubacute Sclerosisng Panencephalitis (SSPE) in Karachi, Pakistan: Proceedings of the Third International Symposium on SSPEVellore, IndiaChristian Medical College1989
- ManningLLamanMEdoniHSubacute sclerosing panencephalitis in Papua New Guinean children: the cost of continuing inadequate measles vaccine coveragePLoS Negl Trop Dis201151e93221245918
- BuchananRBonthiusDJMeasles virus and associated central nervous system sequelaeSemin Pediatr Neurol201219310711422889539
- SantoshkumarBRadhakrishnanKSubstantial spontaneous long-term remission in subacute sclerosing panencephalitis (SSPE)J Neurol Sci1998154183889543327
- DykenPSubacute sclerosing panencephalitis. Current statusNeurol Clin1985311791962581121
- JovićNJEpilepsy in children with subacute sclerosing panencephalitisSrp Arh Celok Lek20131417–843444024073547
- JabbourJDuenasDModlinJSSPE-clinical staging, course, and frequencyArch Neurol1975327493494
- ÖztürkAGürsesCBaykanBGökyiğitAEraksoyMSubacute sclerosing panencephalitis: clinical and magnetic resonance imaging evaluation of 36 patientsJ Child Neurol2002171252911913565
- IchiyamaTClinical course and findings in and differential diagnosis of subacute sclerosing panencephalitisNihon Rinsho20076581481148217695287
- HonarmandSGlaserCAChowESubacute sclerosing panencephalitis in the differential diagnosis of encephalitisNeurology20046381489149315505172
- SarkarNGulatiSDarLBroorSKalraVDiagnostic dilemmas in fulminant subacute sclerosing panencephalitis (SSPE)Indian J Pediatr200471436536715107525
- TripathyKChawlaRMittalKFarmaniaRVenkateshPGulatiSOphthalmic examination as a means to diagnose subacute sclerosing panencephalitis: an optical coherence tomography and ultrawide field imaging evaluationEye Vis2017411
- AndrausMECAndrausCFAlves-LeonSVPeriodic EEG patterns: importance of their recognition and clinical significanceArq Neuropsiquiatr201270214515122311221
- MarkandONPansziJGThe electroencephalogram in subacute sclerosing panencephalitisArch Neurol197532117197261180740
- KatayamaYHottaHNishimuraATatsunoYHommaMDetection of measles virus nucleoprotein mRNA in autopsied brain tissuesJ Gen Virol19957612320132048847530
- Praveen-KumarSSinhaSTalyABThe spectrum of MRI findings in subacute sclerosing panencephalitis with clinical and EEG correlatesJ Pediatr Neurol201192177185
- AkdalGBaklanBÇakmakçiHKovanlikayaAMRI follow-up of basal ganglia involvement in subacute sclerosing panencephalitisPediatr Neurol200124539339511516619
- AlkanASaracKKutluREarly-and late-state subacute sclerosing panencephalitis: chemical shift imaging and single-voxel MR spectroscopyAm J Neuroradiol200324350150612637304
- YükselDDirenBUlubayHAltunbaşakŞAnlarBNeuronal loss is an early component of subacute sclerosing panencephalitisNeurology2014831093894425085642
- JafriSKHusenYAhmedKIbrahimSHSpectrum of MRI brain findings in subacute sclerosing panencephalitisAsia Pac J Clin Trials Nerv Syst Dis201724124
- OrensteinWACairnsLHinmanANkowaneBOlivéJMReingoldALMeasles and Rubella Global Strategic Plan 2012–2020 midterm review report: background and summaryVaccine201836A35A4229307368
- RafiqueAAmjadNChandPSubacute sclerosing panencephalitis: clinical and demographic characteristicsJ Coll Physicians Surg Pak201424855756025149833
- KannanLGargSKAryaRSankarMJAnandVTreatments for subacute sclerosing panencephalitisCochrane Database Syst Rev201312CD010867
- BautistaREThe use of the ketogenic diet in a patient with subacute sclerosing panencephalitisSeizure200312317517712651085
- TatlıBEkiciBÖzmenMCurrent therapies and future perspectives in subacute sclerosing panencephalitisExpert Rev2012124485492
- TomodaANomuraKShiraishiSTrial of intraventricular ribavirin therapy for subacute sclerosing panencephalitis in JapanBrain Dev200325751451713129596
