Abstract
Purpose
Pediatric-onset multiple sclerosis (POMS) accounts for ~5% of all multiple sclerosis cases, and has a prevalence of ~10,000 children in the USA. POMS is associated with a higher relapse rate, and results in irreversible disability on average 10 years earlier than adult-onset multiple sclerosis. Other manifestations of POMS include mental and physical fatigue, cognitive impairment, and depression. We believe that the health behaviors of physical activity, diet, and sleep may have potential benefits in POMS, and present a scoping review of the existing literature.
Methods
We identified papers by searching three electronic databases (PubMed, GoogleScholar, and CINAHL). Search terms included: pediatric multiple sclerosis OR pediatric onset multiple sclerosis OR POMS AND health behavior OR physical activity OR sleep OR diet OR nutrition OR obesity. Papers were included in this review if they were published in English, referenced nutrition, diet, obesity, sleep, exercise, or physical activity, and included pediatric-onset multiple sclerosis as a primary population.
Results
Twenty papers were identified via the literature search that addressed health-promoting behaviors in POMS, and 11, 8, and 3 papers focused on diet, activity, and sleep, respectively. Health-promoting behaviors were associated with markers of disease burden in POMS. Physical activity participation was associated with reduced relapse rate, disease burden, and sleep/rest fatigue symptoms. Nutritional factors, particularly vitamin D intake, may be associated with relapse rate. Obesity has been associated with increased risk of developing POMS. POMS is associated with better sleep hygiene, and this may benefit fatigue and quality of life.
Discussion
Participation in health behaviors, particularly physical activity, diet, and sleep, may have benefits for POMS. Nevertheless, there are currently no interventions targeting promotion of these behaviors and examining the benefits of managing the primary and secondary manifestations of POMS.
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Multiple sclerosis (MS) is an immune-mediated disease of the central nervous system that was once considered an adult disease, but can also occur in children and adolescents (i.e., pediatric-onset MS or POMS).Citation1 Indeed, an estimated 3%–5% of adults with MS report experiencing initial symptomatic episodes before the age of 18 years.Citation2,Citation3 We further note that an estimated 10,000 children and adolescents in the USA have been diagnosed with MS, and an additional 15,000 have had symptoms indicative of an MS diagnosis.Citation4
POMS typically presents as a relapsing–remitting clinical course.Citation5 POMS has a slower progression rate than adult-onset MS, but earlier onset does not necessarily portend better long-term prognosis.Citation6 POMS typically transitions into a secondary-progressive clinical course, on an average, 10 years earlier in chronological age than adult-onset MS, presumably based on the earlier disease onset.Citation5,Citation7 We further note that POMS is associated with higher relapse rates than observed in adults with MS,Citation8 and those with POMS reach irreversible and permanent disability earlier in adult life than those with adult-onset MS.Citation5 POMS has many symptomatic manifestations such as mental and physical fatigue,Citation9,Citation10 depression,Citation11 and cognitive impairment.Citation12–Citation15 Such manifestations might have considerable impact on academic performance, social interaction and development, and ultimately quality of life in POMS.
There is a focus on early initiation of treatment after first symptom occurrence of POMS, as this is seemingly associated with better long-term outcomes.Citation16 The first line of treatment for POMS includes injectable therapy with interferon-β and glatiramer acetate.Citation17 The application of these disease-modifying drugs reduces relapse rate and delays disease progression,Citation17 but upward of 30% of children experience breakthrough disease activity and residual symptoms.Citation18 Based on a US cohort of POMS patients evaluated in specialty clinics, over one-fourth of patients prescribed interferon-β discontinued usage because of poor tolerance.Citation19 Since 2010, 3 oral, disease-modifying drugs have been approved as first-line agents for adults with MS, but clinical trials of these agents for POMS are either in process of enrollment (teriflunimide, dimethyl fumarate) or nearing completion (fingolimod). Importantly, no pharmacological treatment, to date, has been approved by the US Food and Drug Administration for POMS. Furthermore, there is a paucity of data on disease-modifying drugs effect on functional and symptomatic outcomes in POMS.Citation19
We believe it is time that researchers and clinicians focus on health behaviors as an approach for managing the manifestations, and perhaps course, of POMS. This argument is based on evidence from clinical trials that health behaviors exert beneficial effects on dysfunction, symptoms, and participation outcomes and perhaps disease progression among adults with MS.Citation20 “Health behavior” is defined as specific actions an individual performs for health management, maintenance, and restoration combined with the determinants, correlates, and consequences of those actions.Citation21 Health behavior can be described as participation in directly observable actions that broadly influence health status (i.e., state of physical, mental, and social well-being). Health behavior can be separated into 2 distinct categories, namely health-promoting and health-compromising behaviors.Citation22 Health-promoting behaviors benefit or improve health, and health-compromising behaviors weaken health in either the short or long term. Health-promoting behaviors typically involve physical activity, including exercise; healthy diet, including weight control; and sleep hygiene, including consistent bedtime routines.Citation22
We undertook a scoping review of health behaviors in POMS, as it provided an overview or landscape of current evidence and identified future directions for health behavior research in this population. We limited our focus on the health behaviors of diet, physical activity, and sleep as these 3 health behaviors are most commonly studied in POMS, and further represent appropriate targets for future health promoting, behavioral interventions. Obesity is included in diet, as obesity is frequently an outcome of health-compromising behaviors, such as overeating. This scoping review is a necessary first step in 1) establishing a research agenda for health behavior participation, 2) developing health promotion programs, and 3) creating population-specific interventions targeting health behavior engagement (i.e., physical activity, nutrition, and sleep) within POMS.
Methods
Papers related to health behavior participation in youth with POMS were identified by searching 3 electronic databases (i.e., PubMed, GoogleScholar, and CINAHL) using scoping review procedures.Citation23,Citation24 The papers were included from the timeframe of 1984 (i.e., date of the earliest known published paper on POMS)Citation25 through October of 2017.
Eligibility
Studies were included in this review based on the following criteria: 1) POMS was a primary population of interest; 2) nutrition, diet, obesity, physical activity, exercise, or sleep were identified as focal health behaviors; and 3) the paper was published in English. Two abstracts were included as both provided novel information with regard to health behavior in POMS and have been cited in systematic and scoping reviews. Book chapters were excluded.
Data extraction
The preliminary literature search was undertaken in August 2017, with a follow-up search in October 2017. One author (EMS) conducted the search and retrieved the papers from databases. Searches were conducted by using the following search phrases: pediatric multiple sclerosis OR pediatric onset multiple sclerosis OR POMS AND health behavior OR physical activity OR sleep OR diet OR nutrition OR obesity. Duplicate records were excluded. Studies were included based on a review of the Abstract, Introduction, and Discussion sections. One author (EMS) manually searched reference lists for identifying additional papers not captured in the electronic literature search.
Data synthesis/analysis
Papers were coded into categories by 1 author (EMS) after review. Papers were categorized as investigating nutrition/diet/obesity, exercise/physical activity, or sleep. Two papers discussed 2 of the health behaviors and were sorted into 2 categories. Subcategories within each category emerged during the coding process. For example, sodium intake emerged as a subcategory of nutrition/diet/obesity because it was identified in several papers within this category. Title, author, primary results, and targeted health behavior data were extracted and synthesized into tables. – contain specific information for diet, exercise, and sleep, respectively.
Table 1 Papers investigating diet/nutrition/obesity
Table 2 Papers investigating physical activity
Table 3 Papers investigating sleep
Results
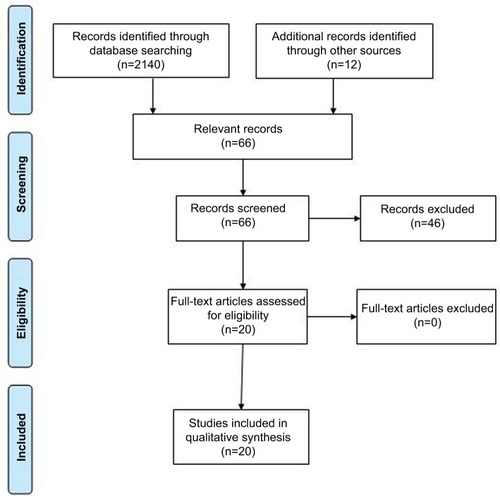
One reviewer (EMS) identified 66 relevant papers through the electronic literature search and manual inspection of references. Of those, 20 papers remained after duplicates were removed via title search performed in Microsoft Word (). Of the papers identified during this review, 11 involved diet, nutrition, or obesity; 8 physical activity; and 3 sleep.
Diet/nutrition
Most papers in this category were case–control studies (6 of 11).Citation26–Citation31 Three were retrospective observational studiesCitation32–Citation34 and 2 prospective longitudinal studies.Citation35,Citation36
Factors associated with nutrition comprised 7 of the 11 papers included in this section. Four papers investigated vitamin D status and its association with POMS.Citation31–Citation33,Citation36 One reported no difference in the prevalence of insufficiency (p=0.98) or deficiency (p=0.81) in vitamin D between children with POMS and adults with MS.Citation32 Two papers suggested that vitamin D levels were associated with relapse rate (p=0.03 and p=0.02)Citation33,Citation36 such that higher levels of vitamin D were associated with fewer relapses in POMS. One of those papers reported that the association between vitamin D and relapse rate is mediated by a known genetic MS risk factor (e.g., human leukocyte antigen DRB1*15:01 or 15:03).Citation36 Adequate vitamin D3 status was associated with a 34% decrease in subsequent relapse rates.Citation33 The fourth paper reported that the vitamin D genetic risk score was associated with a decreased risk of developing MS (p=0.02).Citation31
Two papers investigated the association between iron and POMS.Citation27,Citation35 One paper reported no association between iron intake and risk of relapse for children with POMS.Citation35 The other paper reported that dietary iron was lower in POMS than controls (p=0.04), and POMS was associated with a reduction in the consumption of recommended amounts of iron (p<0.01). The paper further reported that iron consumption below the recommended amount was associated with diagnosis of POMS (p<0.01).Citation27 Two papers investigated the role of sodium in POMS.Citation26,Citation30 One paper reported no association between sodium intake and POMS diagnosis (p=0.93), and no significant difference in sodium intake between POMS and healthy controls (p=0.99).Citation26 The second paper reported that sodium was not associated with time to subsequent relapse, and children with POMS who had higher sodium intake did not have less time between relapses compared with those with low sodium intake (medium sodium intake compared with low, hazard ratio [HR]=0.69, high sodium intake compared with low, HR=1.37).Citation30
Six of the 11 papers focused on obesity.Citation28,Citation29,Citation31,Citation32,Citation34,Citation35 Three papers identified high body mass index or obesity as a risk factor for developing POMS.Citation28,Citation29,Citation31 One of those papers reported that obesity was associated with a higher risk for developing POMS among girls than boys,Citation29 but the other paper reported that it was a risk factor for both sexes.Citation28 One case–control study reported that body mass index was higher in children diagnosed with POMS than in controls, and higher body mass index was further associated with younger onset of symptoms.Citation28 Another study reported that children with POMS were more obese than adults with MS, independent of gender.Citation32 One final paper reported that over 50% of children were overweight or obese at the time of POMS diagnosis.Citation34
Physical activity
Most papers in this category included a cross-sectional research design (4 of 8).Citation37–Citation40 Two of the cross-sectional reports were printed as abstract only.Citation39,Citation40 Two papers were reviewed,Citation19,Citation41 one was an editorial publicationCitation42 and the other was a validation study.Citation43
Four of the 8 studies indicated that children diagnosed with POMS engaged in less physical activity than healthy controls and 2 other types of demyelinating disease (i.e., monophasic acquired demyelinating syndrome or clinically isolated syndrome).Citation37,Citation38,Citation40,Citation43 The magnitude of difference ranged between d=0.02 and d=0.53, as measured by accelerometer, and d=0.00 to d=0.73, as measured by self-report (Godin Leisure-Time Exercise Questionnaire). Of note, the 2 abstracts focusing on physical activity in POMS did not provide enough information to calculate effect sizes. Two papers investigated correlates of physical activity in POMS. One paper reported that exercise goal setting and self-efficacy were associated with physical activityCitation38 and the other reported self-efficacy and functional disability were correlated with self-report and objective measures of physical activity.Citation39 Those with POMS who reported lower exercise goal setting and self-efficacy had lower levels of physical activity, as did those with higher perceived functional limitations. Two papers noted that physical activity and exercise were appropriate methods for managing fatigue,Citation20,Citation44 and 2 papers suggested that physical activity participation may influence disease burden and outcomes for those with POMS.Citation37,Citation42 For example, physical activity, particularly participation in strenuous exercise, was associated with smaller T2 lesion volumes in a sample of subjects with POMS. Another study reported that those with POMS who engaged in higher levels of physical activity reported less fatigue, fewer sleep/rest fatigue symptoms, and lower relapse rates.Citation37,Citation38 On the other hand, no associations were reported between physical activity and total brain volume.Citation37
Sleep in POMS
One paper suggested that children diagnosed with POMS are more successful with managing sleep hygiene (e.g., followed consistent bedtime routines) than age-, sex-, and race-matched healthy controls.Citation45 This information was based on the Adolescent Sleep Hygiene Scale, which provides a total hygiene score based on physiological, cognitive, emotional, sleep environment, substances, and sleep stability subscales. The children with POMS scored higher than matched controls in the sleep stability subscale, which evaluates the frequency of adherence to regular sleep times on weekends and weekdays. Two other papers reported that those with POMS who participated in higher levels of physical activity reported less sleep/rest fatigue symptoms than those who did not achieve adequate levels of physical activity.Citation37,Citation38
Discussion
We performed a scoping review of research on diet, physical activity, and sleep as health behaviors of importance in POMS. Of those health behaviors, diet and nutritional factors have received the most research attention in POMS. Results from this review indicate that 1) dietary factors and obesity are related to risk of developing POMS, 2) vitamin D intake is related to relapse rate, and 3) children with POMS are more obese than both healthy controls and adults with MS. Vitamin D supplements are frequently recommended for children with POMS, but there are no interventions targeting diet or nutrition for this group to date. Vitamin D represents an interesting and exciting avenue for intervention as relapse rates are twice as high in POMS as in adults with MS. Obesity is thought to be a consequence of health- compromising behaviors (i.e., physical inactivity, nutritional insufficiency, over consumption of energy). Obesity further is a commonly identified concern for youth with POMS, but there are no studies to date that have investigated strategies to influence diet, nutrition, or physical activity participation as causes of obesity. Campaigns to prevent obesity are prevalent in the USA, and many have been deemed effective in terms of preventing and reducing obesity.Citation46 This information has not yet been translated to youth with POMS, but this group would benefit from these types of interventions in the future.
The second most common focus of research on health behaviors in POMS involved physical activity. Collectively, the 3 observations from this body of research include 1) there is reduced physical activity participation in POMS; 2) goal-setting, self-efficacy, and perceived limitations represented Social Cognitive Theory correlates of physical activity in POMS; and 3) physical activity might be associated with markers of disease burden and symptomology. Such observations are important as physical activity represents a modifiable factor for symptom management in POMS. There are currently no interventions designed to target physical activity participation for this group. Future research is required to identify appropriate study designs and intervention strategies, as well as measure outcomes of such interventions on primary and secondary symptoms of POMS.
With regard to the measurement of physical activity for future interventions, accelerometry provides 1 method that is objective and non-biased. Unfortunately, accelerometers are unable to capture all types of physical activities (i.e., swimming and cycling) due to required placement on the body and/or water resistance issues with the devices. There has been a single self-reported measure of physical activity (Godin Leisure-Time Exercise Questionnaire) validated for use in research and clinical practice with children diagnosed with POMS,Citation43 and it is a useful tool for capturing those types of physical activity behaviors that are not captured by accelerometry. The Godin Leisure-Time Exercise Questionnaire is a simple, effective, and useful tool that may be utilized in clinical environments and research to measure physical activity and intervention outcomes. Thus, future interventions designed to promote physical activity behaviors may utilize this validated measure.
With regard to sleep, only 1 paper focused on this health behavior as a primary variable of interest, and 2 papers included it as a secondary endpoint. Youth with POMS demonstrated more appropriate methods for sleep hygiene than healthy peers. This is an interesting factor to consider. Perhaps, children with POMS unconsciously regulate sleep patterns to adjust for daytime fatigue or sleepiness. Another factor for this improved sleep self-management may be familial involvement or scheduling. As children and adolescents frequently follow schedules set by family or caregivers, these consistent bedtime routines may be a result of habits learned at earlier ages. The aforementioned paper did not measure the average amount of sleep this population obtains, but previous data with regard to all children in the USA report inadequate amounts of sleep. A poll by the National Sleep Foundation in 2014 reported that children between the ages of 13 and 17 years old average 7 hours sleep per night, based on parental report, but between 8 and 10 hours per night is recommended for this age range by the American Academy of Sleep Medicine.Citation47 Previous research further indicates children with other chronic illnesses exhibit higher rates of sleep problems, including frequent nighttime wakening and problems falling asleep.Citation48 Future research is required to determine average amount of sleep obtained by youth with POMS, and the impact of this amount and quality of sleep.
Limitations of the research
As in all reviews, there is the potential for missing literature published after the search was conducted, but we repeated the search immediately before finishing this paper. Only 1 author conducted the search and reviewed papers, reducing the option for a second opinion with regard to inclusion and data extraction; all authors discussed the characterization of results and description of all the studies. The relatively small number of papers located, combined with the generally small sample sizes, limit the generalizability of results and, perhaps, the precision of the findings per paper and overall.
Unfortunately, there is very little research on health behaviors, particularly outside of diet/nutrition and physical activity, in youth with POMS, and published papers primarily investigate the etiologic role of health behavior in disease development and progression. There is substantially less known about the effect of health behaviors on secondary manifestations of POMS such as symptoms, function, quality of life, academic performance, and participation.
Future directions
The possible benefits of health behavior engagement have not been thoroughly vetted in POMS. We believe there is an abundant opportunity for descriptive research on healthy behaviors in POMS (e.g., what are common health behaviors in POMS?); validation research on appropriate methods for measuring health behaviors (e.g., diary vs questionnaire) and the reliability of such measures; qualitative and cross-sectional research on predictors (e.g., self-efficacy and goal setting) and consequences (e.g., reduced relapse rates and increased quality of life) of health behaviors in POMS; longitudinal research on changes in health behaviors over time (e.g., youth with POMS participate in higher amounts of physical activity in the summer compared with the winter) and associated predictors and consequences; and experimental research testing the feasibility and efficacy on interventions for changing health behaviors and examining the effects on disease and symptomatic outcomes in POMS. One particularly important focus might involve the effect of health behavior interventions on psychological distress in POMS, but there is currently limited research in this area in the literature. If researchers systematically address these opportunities, clinicians will have a greatly expanded body of research for supporting the promotion of health behavior change in the management of POMS.
Conclusion
Overall, the secondary and residual symptoms associated with POMS may be managed effectively by health behavior participation and self-management, as has been described in adults with MS.Citation49 Beyond the potential for beneficial effects of health behavior on disease and symptom outcomes for those with POMS, health behavior participation in adolescence likely tracks into adulthood.Citation50 For example, youth who engage in health-promoting behaviors like regular physical activity, appropriate nutrition, and good sleep patterns often continue this behavior into adulthood. To that end, the potential benefits of health behavior promotion may directly address the needs of POMS and provide a clinical opportunity toward establishing and supporting health behavior habits that track throughout the lifespan among those with MS. We collectively and anxiously await research that systematically addresses health behavior in POMS – this represents an exciting opportunity for empowering self-managing through health behavior change among children and adolescents living with MS.
Disclosure
The authors report no conflicts of interest in this work.
References
- FoxRJBaconTEChamotEPrevalence of multiple sclerosis symptoms across lifespan: data from the NARCOMS RegistryNeurodegener Dis Manag201556 Suppl31026611264
- ChitnisTGlanzBJaffinSHealyBDemographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United StatesMult Scler200915562763119299440
- WaldmanAGhezziABar-OrAMikaeloffYTardieuMBanwellBMultiple sclerosis in children: an update on clinical diagnosis, therapeutic strategies, and researchLancet Neurol201413993694825142460
- National Multiple Sclerosis Society [cited 2016]. Available from: https://www.nationalmssociety.org/What-is-MS/Who-Gets-MS/Pediatric-MSAccessed November 21, 2017
- BoikoAVorobeychikGPatyDDevonshireVSadovnickDUniversity of British Columbia MS Clinic NeurologistsEarly onset multiple sclerosis: a longitudinal studyNeurology20025971006101012370453
- RenouxCVukusicSMikaeloffYAdult Neurology Departments KIDMUS Study GroupNatural history of multiple sclerosis with childhood onsetN Engl J Med2007356252603261317582070
- HardingKELiangKCossburnMDLong-term outcome of paediatric-onset multiple sclerosis: a population-based studyJ Neurol Neurosurg Psychiatry201384214114723154123
- GormanMPHealyBCPolgar-TurcsanyiMChitnisTIncreased relapse rate in pediatric-onset compared with adult-onset multiple sclerosisArch Neurol2009661545919139299
- CarrollSChalderTHemingwayCHeymanIMoss-MorrisR“It feels like wearing a giant sandbag.” Adolescent and parent perceptions of fatigue in paediatric multiple sclerosisEur J Paediatr Neurol201620693894527422092
- CarrollSChalderTHemingwayCHeymanIMoss-MorrisRUnderstanding fatigue in paediatric multiple sclerosis: a systematic review of clinical and psychosocial factorsDev Med Child Neurol201658322923926566789
- ParrishJBWeinstock-GuttmanBSmerbeckABenedictRHYehEAFatigue and depression in children with demyelinating disordersJ Child Neurol201328671371822805247
- BaruchNFO’DonnellEHGlanzBICognitive and patient-reported outcomes in adults with pediatric-onset multiple sclerosisMult Scler201622335436126041802
- Nunan-SaahJPaulrajSRWaubantEKruppLBGomezRGNeuro-psychological correlates of multiple sclerosis across the lifespanMult Scler201521111355136426163074
- MacAllisterWSBelmanALMilazzoMCognitive functioning in children and adolescents with multiple sclerosisNeurology20056481422142515851734
- MacAllisterWSBoydJRHollandNJMilazzoMCKruppLBInternational Pediatric MS Study GroupThe psychosocial consequences of pediatric multiple sclerosisNeurology20076816 Suppl 2S66S6917438240
- KavaliunasAManouchehriniaAStawiarzLImportance of early treatment initiation in the clinical course of multiple sclerosisMult Scler20172391233124027754943
- GhezziAAmatoMPMakhaniNShreinerTGärtnerJTenembaumSPediatric multiple sclerosis conventional first-line treatment and general managementNeurology2016879 Suppl 2S97S10227572869
- ChitnisTGhezziABajer-KornekBBoykoAGiovannoniGPohlDPediatric multiple sclerosis Escalation and emerging treatmentsNeurology2016879 Suppl 2S103S10927572854
- YehEAManagement of children with multiple sclerosisPaediatr Drugs201214316517722497553
- MotlRWPiluttiLAIs physical exercise a multiple sclerosis disease modifying treatment?Expert Rev Neurother201616895196027219279
- GlanzKRimerBViswanathThe scope of health behaviorGlanzRimerViswanathHealth Behavior and Health Education: Theory, Research, and PracticeSan Francisco, CAJossey-Bass2008322
- SchwarzerRModeling health behavior change: how to predict and modify the adoption and maintenance of health behaviorsAppl Psychol2008571129
- ArkseyHO’MalleyLScoping studies: towards a methodological frameworkInt J Soc Res Methodol2005811932
- ColquhounHLLevacDO’BrienKKScoping reviews: time for clarity in definition, methods, and reportingJ Clin Epidemiol201467121291129425034198
- IshiharaOYamaguchiYMatsuishiTMultiple ring enhancement in a case of acute reversible demyelinating disease in childhood suggestive of acute multiple sclerosisBrain Dev1984644014066496875
- McDonaldJGravesJWaldmanAA case-control study of dietary salt intake in pediatric-onset multiple sclerosisMult Scler Relat Disord20166879227063630
- PakpoorJSeminatoreBGravesJSUS Network of Pediatric Multiple Sclerosis CentersDietary factors and pediatric multiple sclerosis: a case-control studyMult Scler2017 1352458517713343
- ChitnisTGravesJWeinstock-GuttmanBDistinct effects of obesity and puberty on risk and age at onset of pediatric MSAnn Clin Transl Neurol201631289790728097202
- Langer-GouldABraraSMBeaberBEKoebnickCChildhood obesity and risk of pediatric multiple sclerosis and clinically isolated syndromeNeurology201380654855223365063
- NourbakhshBGravesJCasperTCNetwork of Pediatric Multiple Sclerosis CentersDietary salt intake and time to relapse in paediatric multiple sclerosisJ Neurol Neurosurg Psychiatry201687121350135327343226
- GianfrancescoMAStridhPRheadBNetwork of Pediatric Multiple Sclerosis CentersEvidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MSNeurology201788171623162928356466
- BrentonJNKoenigSGoldmanMDVitamin D status and age of onset of demyelinating diseaseMult Scler Relat Disord20143668468825891547
- MowryEMKruppLBMilazzoMVitamin D status is associated with relapse rate in pediatric-onset multiple sclerosisAnn Neurol201067561862420437559
- KryskoKYehEAHanwellHCohenARotsteinDObesity and disease activity in pediatric-onset multiple sclerosis (P1376)Neurology20168616 Suppl376
- AzarySSchreinerTGravesJContribution of dietary intake to relapse rate in early paediatric multiple sclerosisJ Neurol Neurosurg Psychiatry2018891283328993476
- GravesJSBarcellosLFShaoXU.S. Network of Pediatric MS CentersGenetic predictors of relapse rate in pediatric MSMult Scler J2016221215281535
- GroverSAAubert-BrocheBFetcoDLower physical activity is associated with higher disease burden in pediatric multiple sclerosisNeurology201585191663166926268901
- GroverSASawickiCPKinnett-HopkinsDPhysical activity and its correlates in youth with multiple sclerosisJ Pediatr201617919720327717498
- SawickiCGroverSKinnett-HopkinsDSelf-efficacy and functional disability as barriers to physical activity participation in pediatric multiple sclerosis (P4.023)Neurology20158414 Suppl P4. 023
- GroverSAKinnett-HopkinsDSawickiCPLow levels of participation in vigorous physical activity in youth with multiple sclerosis and the associations with fatigue and depressionMultiple Sclerosis Journal201521379380
- YehEAKinnett-HopkinsDGroverSAMotlRWPhysical activity and pediatric multiple sclerosis: developing a research agendaMult Scler201521131618162526447061
- RoccaMAFilippiMDeivaKPromoting physical activity to control multiple sclerosis from childhoodNeurology201585191644164526268902
- Kinnett-HopkinsDGroverSAYehEAMotlRWPhysical activity in pediatric onset multiple sclerosis: Validating a questionnaire for clinical practice and researchMult Scler Relat Disord201610262927919494
- YehEAWeinstock-GuttmanBThe management of pediatric multiple sclerosisJ Child Neurol201227111384139322914373
- ZafarABNessJDowdySAvisKBashirKExamining sleep, fatigue, and daytime sleepiness in pediatric multiple sclerosis patientsMult Scler201218448148821965417
- SticeEShawHMartiCNA meta-analytic review of obesity prevention programs for children and adolescents: the skinny on interventions that workPsychol Bull2006132566769116910747
- ParuthiSBrooksLJD’AmbrosioCRecommended amount of sleep for pediatric populations: a consensus statement of the American Academy of Sleep MedicineJ Clin Sleep Med201612678578627250809
- HysingMSivertsenBStormarkKMElgenILundervoldAJSleep in children with chronic illness, and the relation to emotional and behavioral problems-a population-based studyJ Pediatr Psychol200834666567018786977
- MotlRWSandroffBMKwakkelGExercise in patients with multiple sclerosisLancet Neurol2017161084885628920890
- LauRRQuadrelMJHartmanKADevelopment and change of young adults’ preventive health beliefs and behavior: influence from parents and peersJ Health Soc Behav19903132402592133479