Abstract
In acromegaly, achieving biochemical control (growth hormone [GH] level <1.0 ng/mL and age- and sex-normalized levels of insulin-like growth factor 1 [IGF-1]) through timely diagnosis and appropriate treatment provides an opportunity to improve patient outcomes. Diagnosis of acromegaly is challenging because it is rooted in observing subtle clinical manifestations, and it is typical for acromegaly to evolve for up to 10 years before it is recognized. This results in chronic exposure to elevated levels of GH and IGF-1 and delay in patients receiving appropriate treatment, which consequently increases mortality risk. In this review, the clinical impact of elevated GH and IGF-1 levels, the effectiveness of current therapies, and the potential role of novel treatments for acromegaly will be discussed. Clinical burden of acromegaly and benefits associated with management of GH and IGF-1 levels will be reviewed. Major treatment paradigms in acromegaly include surgery, medical therapy, and radiotherapy. With medical therapies, such as somatostatin analogs, dopamine agonists, and GH receptor antagonists, a substantial proportion of patients achieve reduced GH and normalized IGF-1 levels. In addition, signs and symptoms, quality of life, and comorbidities have also been reported to improve to varying degrees in patients who achieve biochemical control. Currently, there are several innovative therapies in development to improve patient outcomes, patient use, and access. Timely biochemical control of acromegaly ensures that the patient can ultimately improve morbidity and mortality from this disease and its extensive consequences.
Introduction
Acromegaly is a rare and chronic disease characterized most often by hypersecretion of growth hormone (GH) from benign somatotrophic adenomas.Citation1 Hypersecretion of GH stimulates excess production of insulin-like growth factor 1 (IGF-1) from the liver and systemic tissues.Citation2,Citation3 Earlier estimates of the acromegaly annual incidence rate of three to four cases per million per year and the prevalence rate of ~70 total cases per millionCitation4 were recently reexamined.Citation5 Recent estimates describe a similar overall incidence and prevalence between 2008 and 2012, with an overall incidence at eleven per million person-years and an overall prevalence at 78 cases per million each year. Much higher prevalence of up to 480 cases per million has been reported, although, in that study, 2,270 patients with type 2 diabetes mellitus or glucose intolerance were screened, three were found to have acromegaly, and calculations were made based on the prevalence of type 2 diabetes mellitus and glucose intolerance in the general population and in patients with acromegaly.Citation6 The disease occurs at all ages and is equally distributed between sexes.Citation4 Although there were no differences in incidence or prevalence noted between males and females, increasing age was associated with increased incidence and prevalence.Citation5 Incidence rates were estimated to be between three and eight cases per million person-years in children 0 year to 17 years of age and nine to 18 cases per million person-years in adults >65 years of age. Prevalence rates were estimated to range from 29 to 37 cases per million person-years in children and from 148 to 182 cases per million person-years in adults. The average delay in diagnosing acromegaly appears to be between 4 years and 10 years after the onset of symptoms in adults, as determined from duration of symptoms and alterations in appearance confirmed via photographs.Citation4,Citation5,Citation7
The clinical manifestations of acromegaly result from the pleiotropic effects of increased levels of GH and IGF-1 on many organs, which lead to a multisystem disease associated with various comorbidities, premature mortality, and physical disfigurement.Citation2,Citation3,Citation7,Citation8 The classic signs and symptoms of acromegaly include acral soft-tissue changes, widening teeth spacing, and brow ridge prominence. More commonly consistent patient complaints include excessive perspiration, soft-tissue swelling, large-joint osteoarthritis, and carpal tunnel syndrome.Citation2,Citation7,Citation9 Importantly, patients with acromegaly also develop a significant number of additional comorbidities, which consist of cardiovascular disease (eg, hypertension and cardiomyopathy), insulin resistance, respiratory complications (eg, sleep apnea), and neoplasia (eg, colon polyps).Citation7 Overall, although there have been recent improvements, acromegaly is associated with an increased mortality rate, with GH and IGF-1 levels being important determinants.Citation10,Citation11 Patient age at the time of surgery and treatment with somatostatin analogs (SSAs) is associated with lower mortality. In one study published in 2004, the mean age (± standard deviation [SD]) of patients at initial diagnosis of acromegaly was 42 (±13) years; the mean duration of follow-up (±SD) was 13.4 (±9.9) years; and the mean age (±SD) at death was 61 (±12.8) years.Citation12 The main causes of death were cardiovascular, cerebrovascular, and cancer. Although baseline GH levels were similar between surviving patients and those who had died, surviving patients had significantly lower GH levels at the time of the last follow-up visit (P<0.0001). In general, outcomes have improved with time.Citation13 A recent meta-analysis found that later year of publication was an important efficacy determinant for acromegaly outcomes with treatment.
Currently, there are three treatment options available: surgery, medical therapy, and radiotherapy. The goals of acromegaly treatment include shrinkage or removal of the tumor, safeguarding normal pituitary function, improving symptoms caused by excess GH and IGF-1 levels, including comorbidities and a decrease in mortality risk, and achieving biochemical control.Citation13 According to the most recent acromegaly clinical guidelines from the Endocrine Society (ENDO), biochemical control is defined as achieving a random GH level <1.0 ng/mL and an age-normalized serum IGF-1 level.Citation3 Achieving these recommended biochemical target goals has been shown to correlate with reduced mortality risk and improvement in clinical symptoms and outcomes in patients with acromegaly. This review discusses the clinical evidence and guideline recommendations for current medical therapies used to achieve biochemical control, the importance of biochemical control in patients with acromegaly, and the potential role of investigational medical therapies in the treatment of patients with acromegaly.
Medical therapies for biochemical control
Transsphenoidal surgery is recommended as the primary therapy in most patients with acromegaly.Citation3 Remission had been defined as achieving a normal IGF-1 level and a GH level <1.0 ng/mL during a glucose load (oral glucose tolerance test [OGTT]).Citation14 Advances in the sensitivity of assays led to revising recommendations to achieve normal IGF-1, GH <0.4 ng/mL during glucose load, or a random GH <1.0 ng/mL.Citation14,Citation15 A lack of consensus in the field for particular criteria has led to a lack of consistent remission rate reporting.Citation14–Citation16 What is clear is that although surgical success is reported in many patients, some do not achieve adequate control of disease after surgery; this lack of biochemical control is characterized by persistently elevated GH and IGF-1 levels. Thus, in these patients with persistent or recurrent disease, additional therapeutic intervention such as medical therapy is needed to improve patient outcomes.
Medical therapy is recommended in patients who are poor surgical candidates and in those who did not achieve biochemical remission with surgery.Citation3 Medical therapy has also been considered for use preoperatively to improve surgical outcomes. When using a criterion of GH nadir <1.0 ng/mL during OGTT, pretreatment with medical therapy for 3–6 months increased surgical cure rates from 15% to 18% in the control groups receiving surgery alone and to 38% to 42% in the groups receiving medical treatment and surgery.Citation17–Citation19
Three different medical therapy approaches can be taken to address control of GH and IGF-1, including stimulation of somatostatin receptors (SSA), inhibition of GH receptors (GH receptor antagonist [GHRA]), and stimulation of dopamine receptors (dopamine agonist [DA]; ).
Figure 1 Classes of medical therapy providing biochemical control for patients with acromegaly.
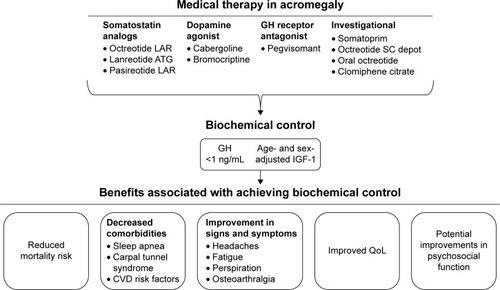
Somatostatin analogs
Somatostatin, also known as GH-inhibiting hormone, inhibits the release of GH from the pituitary.Citation20 SSAs, which are synthetic compounds that mimic activity of endogenous somatostatin receptor (sst) ligand, are considered a mainstay of acromegaly therapy.Citation13,Citation20 There are five subtypes of ssts, with sst2 and sst5 being the predominantly expressed ssts in GH-secreting pituitary adenomas.Citation2,Citation7,Citation21 Several SSAs are currently approved for the treatment of acromegaly, including octreotide long-acting release (LAR), octreotide subcutaneous (SC), lanreotide Autogel® (ATG), and pasireotide LAR. Octreotide and lanreotide are considered first-generation SSAs, whereas pasireotide is a next-generation, multireceptor-targeted SSA.Citation3,Citation22
In the ENDO clinical guidelines, medical therapy is recommended in patients with persistent disease following surgery.Citation3 The results of a meta-analysis in acromegaly showed that approximately one-half of patients achieved control of GH and/or IGF-1 levels with either octreotide or lanreotide, with longer treatment duration having an appreciable effect on GH levels.Citation23 In addition to improved GH and/or IGF-1 levels, up to 97% of patients treated with octreotide or lanreotide gained control of tumor growth.Citation23,Citation24 It has also been reported that tumor shrinkage occurred more frequently in patients treated with octreotide LAR as primary therapy than as secondary therapy; however, cautious interpretation is warranted as patients in these two different treatment groups were not matched for baseline tumor size.Citation23
The benefits associated with preoperative SSA therapy remain unclear.Citation25,Citation26 Although several small studies suggested a better surgical outcome in patients with invasive macroadenomas who were pretreated with SSAs, other larger studies have not reported a significant difference.Citation26 Thus, recent ENDO clinical guidelines recommend that preoperative therapy should not be used to improve biochemical control after surgery.Citation3
Pasireotide LAR is a next-generation SSA that has demonstrated efficacy in patients with acromegaly and is indicated for the treatment of patients with acromegaly who have had inadequate response to surgery and/or in whom surgery is not an option.Citation23 Unlike octreotide or lanreotide, which primarily exert their effects through binding to sst2, pasireotide binds with high affinity to sst5.Citation27 In a Phase III study, 31.3% of medically naïve patients with acromegaly achieved biochemical control after 12 months of treatment with pasireotide LAR compared with 19.2% of patients treated with octreotide LAR.Citation28 Tumor volume shrinkage and improvements in symptoms and quality of life (QoL) were similar between treatment groups. In another Phase III study in patients with inadequately controlled acromegaly despite treatment with octreotide LAR or lanreotide ATG, 15% and 20% of patients achieved biochemical control with pasireotide LAR 40 mg and 60 mg, respectively, compared with 0% of patients who continued treatment with octreotide LAR or lanreotide ATG.Citation29 Tumor volume reduction (>25%) was also greater in those treated with pasireotide LAR (40 mg, 18.5%; 60 mg, 10.8%) than in those who continued treatment with the active control (1.5%). In addition, more patients in the pasireotide LAR group had improvements in symptom severity scores than those in the active control group. As demonstrated in both Phase III studies, pasireotide LAR has a safety profile that was similar to that of the other SSAs used, except for a higher frequency of hyperglycemia.Citation28,Citation29
GH receptor antagonists
GHRAs block binding of endogenous GH to its receptor and consequently inhibit the secretion of IGF-1 from the liver.Citation7,Citation30 Because GHRAs inhibit the action of GH but not its secretion, GH concentrations should not be used to evaluate treatment efficacy.Citation7 Therefore, IGF-1 should be used as a surrogate marker to assess treatment outcomes. Pegvisomant is indicated for patients with acromegaly who have had an inadequate response to surgery or radiotherapy or for whom these therapies are not appropriate.Citation31 ENDO guidelines recommend that pegvisomant be used in patients with moderate-to-severe acromegaly who have inadequately responded to SSAs.Citation3 In a 12-week study, a greater number of patients with acromegaly achieved normalized IGF-1 levels with pegvisomant in a dose-dependent manner (10 mg, 54%; 15 mg, 81%; 20 mg, 89%) compared with placebo (10%).Citation30 Furthermore, improved scores for signs and symptoms, including soft-tissue swelling, excessive perspiration, fatigue, and ring size, were also observed with pegvisomant compared with placebo. A long-term study of pegvisomant treatment for 5 years demonstrated that IGF-1 levels were normalized in 63.2% of patients with acromegaly.Citation32 In contrast to the pituitary-targeted SSAs, pegvisomant does not have tumor suppressive effects.Citation22 In fact, tumor growth has been reported in 3% of patients treated with pegvisomant, although whether this is related to the drug or to the natural history of the tumor remains to be further elucidated.Citation32,Citation33 Therefore, GHRAs provide an alternate option for achieving control of IGF-1, especially when control is not achieved with an SSA.
Dopamine agonists
Dopamine is a catecholamine neurotransmitter that binds to dopamine receptors expressed in cells of the anterior pituitary gland.Citation34 Bromocriptine and cabergoline are currently available DAs for the treatment of acromegaly.Citation35 Cabergoline, which is limited to use in patients with acromegaly who have modest elevations in IGF-1 levels and mild signs and symptoms of GH excess,Citation3 has a longer half-life and higher selectivity for D2 receptors than bromocriptine. Although cabergoline as a single-agent therapy has modest efficacy in acromegaly, it can often serve as an effective combination therapy with SSAs for achieving full biochemical normalization.
Monitoring success of therapy
Up to 45% of patients do not achieve biochemical control with SSA monotherapy.Citation13 Reasons for lack of response can include diminished expression or mutation of the ssts, specific genetic mutations, or alterations to intracellular signaling pathways.Citation36 Reduction in GH levels in patients with acromegaly in response to a selective sst2 antagonist was directly correlated with expression of sst2, suggesting that receptor density is directly correlated with response.Citation37 In addition, expression of mutated sst5 was negatively correlated with SSA effect, implying that a loss of functional receptor translates to a loss of response.Citation21,Citation37 Alterations to intracellular signaling pathways downstream of sst2 also affect the ability of the patient to respond to SSA therapy. Patients with genetic-mutated aryl hydrocarbon receptor-interacting protein (AIP) gene are resistant to SSA treatment,Citation38 whereas mutations in IGF-binding protein 3 are not correlated with response to therapy.Citation39 Therefore, given that there are various alterations that may affect SSA-mediated biochemical control, close monitoring of response to medical therapy is critical to ensure appropriate medical management.
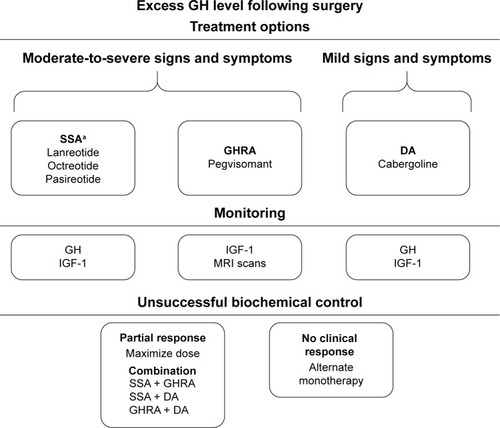
Recommendations for monitoring successful biochemical control vary depending on the medical treatment option chosen (). Guidelines recommend monitoring serum GH and IGF-1 levels when either an SSA or a cabergoline is used, whereas IGF-1 levels and an MRI are recommended with pegvisomant administration.Citation3 Lack of suppression of GH to <1 ng/mL following an OGTT is recommended for diagnostic confirmation of acromegaly in the presence of equivocal serum IGF-1 levels and following surgery. However, assessing GH suppression after an OGTT in patients with acromegaly who have type 2 diabetes mellitus is not recommended, as the OGTT is unreliable.Citation40 Interpretation of test results is not always straightforward because of biological and assay variability. Levels of IGF-1 have to be interpreted in the context of age. Not only is GH secretion episodic, but there is also considerable variability among GH assays.Citation3 Even within one testing center using the same assay, GH levels can vary, making it a challenge to define a value that clearly defines normal. Therefore, to understand biochemical control, it is important to maintain the same GH assay for the same patient throughout management.
Figure 2 Medical management of acromegaly.
Abbreviations: DA, dopamine agonist; GH, growth hormone; GHRA, growth hormone receptor antagonist; IGF-1, insulin-like growth factor 1; MRI, magnetic resonance imaging; SSA, somatostatin analog.
Combination therapy
Alternate strategies should be considered if biochemical control is not achieved with monotherapy. Combining different therapies, especially therapies that target different mechanisms of GH and IGF-1 secretion, may be an effective strategy to gain further biochemical control in patients with acromegaly. A number of combination therapies have been studied that yielded efficacious results in those who have not responded adequately to medical monotherapy. Recent clinical guidelines recommend the addition of pegvisomant or cabergoline to SSAs for patients with inadequate responses to SSAs.Citation3 In a clinical study, combining pegvisomant with an SSA was shown to normalize IGF-1 levels in 95% of patients.Citation41 In addition, combination therapy with cabergoline and an SSA provided normalization of IGF-1 levels in up to 50% of patients who had developed partial treatment resistance to prior SSA therapy. Combination of cabergoline with pegvisomant has also been considered.Citation42 A prospective trial of cabergoline with low-dose pegvisomant provided normal IGF-1 levels to 68% of patients with acromegaly. These studies highlight that combination therapy could improve efficacy in cases where SSA monotherapy fails to provide adequate biochemical control in patients with acromegaly.
Surgical debulking
In patients who are not candidates for surgery, such as those with parasellar disease, surgical debulking has been shown to improve medical therapy.Citation43–Citation45 A retrospective study found that, prior to surgery, GH and IGF-1 levels were normalized with SSA therapy in 29% and 46% of patients, respectively.Citation43 Following surgical debulking, normal GH levels were seen in 54% of patients and IGF-1 control was observed in 78% of patients with subsequent SSA retreatment. Another study documented a significant improvement in SSA success rate from 10% to 55% of patients obtaining normal GH and IGF-1 levels (P<0.0001) with surgical debulking.Citation45
Importance of biochemical control
Acromegaly is an insidious and slowly progressing disease. Because of the probable lag in disease presentation and diagnosis, exposure to elevated levels of GH and IGF-1 in patients with acromegaly is likely to persist for years in the absence of timely diagnosis and proper treatment. A quick and effective restoration of GH and IGF-1 levels to normal levels is critical for improving patient outcomes. Biochemical control becomes more imperative in juveniles because excess GH exacerbates active linear growth at open epiphyseal growth plates, which leads to gigantism.Citation46 Thus, timely biochemical control is an important treatment goal in the setting of excess GH levels. As previously discussed, different classes of medical therapy could be used as monotherapy or in combination to reduce GH and normalize IGF-1 levels in patients with persistent acromegaly who have failed to achieve controlled disease with surgery. This leads to improved patient outcomes and clinical burden of disease.
Achieving control of GH and IGF-1 levels in patients with acromegaly is important for health-care providers, as this translates to normalization of mortality rates and improvement in some comorbidities. In a retrospective study, an age-adjusted univariate analysis showed that patients with fasting GH levels >10 ng/mL at diagnosis and with heart disease or other malignancies had significantly increased mortality risk.Citation47 A meta-analysis of mortality studies in acromegaly performed by Holdaway et alCitation10 demonstrated that a serum GH level <2.5 µg/L or normalization of IGF-1 level was associated with improvements in mortality risk that were comparable to the mortality risk in the normal population. Along with decreasing mortality, achieving biochemical control has also been shown to be associated with improvements in a number of comorbidities, including respiratory disorders (eg, sleep apnea), skeletal complications (eg, carpal tunnel syndrome), and cardiovascular comorbidities (eg, hypertension).Citation9 Moreover, it has been reported that patients treated with SSAs who achieve only suboptimal or partial control of disease demonstrated significant improvements in various cardiovascular risk markers (eg, reductions in systolic and diastolic blood pressure, total and low-density lipoprotein cholesterol levels, and triglyceride levels).Citation48 However, it is important to note that not all cardiorespiratory comorbidities are reversible upon achieving safe GH and IGF-1 levels.Citation49 Thus, it is imperative that patients with acromegaly are diagnosed in a timely manner if optimal management and treatment of comorbidities associated with disease are to be implemented. Patients should be comprehensively screened at diagnosis and periodically thereafter for any comorbidities that may arise. According to ENDO clinical guidelines, it is recommended that comorbidities also be longitudinally monitored and rigorously managed.Citation3
Beyond GH and IGF-1 target goals, improvements in signs and symptoms and QoL are important parameters of disease control in patients with acromegaly. The chronic and debilitating nature of acromegaly diminishes the patient’s QoLCitation50–Citation53 and psychosocial function.Citation28,Citation29,Citation54 In fact, it has been reported that patients with acromegaly who achieved long-term biochemical control scored worse on assessments of apathy, irritability, anxiety, and depression than did matched controls.Citation55 However, in Phase III studies of patients with acromegaly who were treated with SSAs, patients demonstrated marked improvements in perspiration, fatigue, osteoarthralgia, paresthesia, and headaches, as well as in scores on the acromegaly QoL questionnaire.Citation28,Citation29 In another Phase III study, patients with acromegaly treated with lanreotide ATG demonstrated clinical benefits of treatment, including early and sustained reductions in tumor volume and improvements in biochemical control and QoL.Citation56 Notably, a study of patients with IGF-1 levels within the age-adjusted normal range at baseline who were treated with SSA monotherapy and pegvisomant showed improved QoL in the absence of significant changes in IGF-1 levels, which suggests that IGF-1 levels may not be sufficient for assessing improvement in QoL.Citation57 In contrast, a study demonstrated that scores on the acromegaly QoL questionnaire did not differ between patients with controlled disease and those with uncontrolled disease, although those with controlled disease had significantly better psychological subscale scores for appearance.Citation53 Overall, these observations suggest that chronic exposure to elevated levels of GH and IGF-1 prior to diagnosis and proper treatment of disease could play an important role in the persistence of psychosocial symptoms, profoundly affecting QoL in patients with acromegaly. Although long-term biochemical control remains a targeted goal, implementation of long-term care in these patients may be needed to improve overall patient outcomes.
Despite significant clinical improvements in GH and IGF-1 levels, signs and symptoms of disease, comorbidities, and QoL, which are provided by medical therapy in patients with acromegaly, there remains a need for new agents to improve outcomes in patients with persistent disease. A number of pipeline therapies are currently being investigated to improve biochemical control in patients with acromegaly.
Investigational medical therapies
Somatoprim
Somatoprim is a novel SSA that binds preferentially to sst2, sst4, and sst5 and is currently under investigation for the treatment of acromegaly.Citation58,Citation59 It has been reported to be more potent than octreotide in reducing GH levels in vitro and in suppressing GH in somatotroph adenomas not responding to octreotide.Citation58 Importantly, somatoprim demonstrated low insulin-suppressing activity, which is a possible unwanted side effect of octreotide and lanreotide. Given its unique receptor-binding profile and minimal effects on insulin release, preliminary data suggest that somatoprim could become a new medical therapy to treat patients with uncontrolled acromegaly.
Octreotide SC depot
A challenge with octreotide LAR is that it requires reconstitution and is administered intramuscularly.Citation60 Octreotide SC depot is currently being developed as a long-acting octreotide for treatment of acromegaly and could address practical issues associated with the LAR formulation. Octreotide SC depot is administered subcutaneously as a low-volume, thin-needle injection and uses a proprietary ready-to-use FluidCrystal® Injection depot (Camurus AB, Lund, Sweden) that allows for controlled release of octreotide over extended periods.Citation60,Citation61 In a Phase I study, it has been reported that octreotide SC depot significantly reduced IGF-1 levels after 1–2 weeks of treatment in patients with acromegaly and was well tolerated.Citation61 Octreotide SC depot also provided greater bioavailability and quicker initial reduction in IGF-1 levels as compared with octreotide LAR. Meanwhile, adverse events were reported at greater rates in patients receiving octreotide SC depot compared with those receiving the LAR formulation. Adverse events were generally well tolerated and were characterized primarily by mild-to-moderate gastrointestinal events. The potential convenience of administration and greater suppression of IGF-1 could allow octreotide SC depot to be a viable therapeutic option with certain practical advantages and could provide greater biochemical control to patients with acromegaly.
Oral octreotide
First-generation SSAs (eg, octreotide and lanreotide) require either intramuscular or deep SC injections that could present challenges associated with discomforting injections.Citation62 Oral octreotide capsules (OOCs) are currently being studied as a switch therapy for patients with acromegaly who achieved biochemical control with injectable SSAs. The capsules are composed of a novel transient permeability enhancer formulation that facilitates intestinal absorption of octreotide. In a healthy volunteers study, a single dose of OOC 20 mg decreased basal and GH-releasing hormone-induced GH levels. In a recently completed Phase III study, 13 months of treatment with twice-daily OOC led to decreased GH and IGF-1 levels in 62% of patients with acromegaly compared with 89% of those receiving injectable SSAs at baseline. Most adverse events were mild to moderate in intensity and included gastrointestinal, neurological, and musculoskeletal events, which was consistent with the known safety profile of octreotide. Therefore, OOC may offer advantages over other SSA therapies, specifically injectable agents, including convenience associated with ease of administration, no pain or reactions associated with injections, and reduced number of monthly visits and dependence on health-care workers and/or family members for injections.
Clomiphene citrate
Clomiphene citrate (CC) is an oral selective estrogen receptor modulator that increases secretion of luteinizing hormone and follicle-stimulating hormone, which improves hypogonadism and fertility outcomes.Citation63 Estrogens play a role in reducing IGF-1 in GH-deficient patients under GH replacement. In addition, it has been demonstrated that female patients with acromegaly who have mild elevations in IGF-1 levels can benefit from estrogens, either alone or in combination with SSAs. In a prospective, single-center study, CC was evaluated in male patients with acromegaly (n=16), with particular focus on those with low testosterone levels.Citation63 After 3 months of treatment, CC decreased IGF-1 levels by 41% and provided normal IGF-1 levels to 44% of patients. Additionally, mean total testosterone levels increased by 209% in ten patients treated with CC, and normal testosterone levels were achieved in 67% of patients considered to be hypogonadal. No major side effects were reported. CC could be considered as a therapeutic option in patients with acromegaly who are not controlled by the currently available options.
Conclusion
Long-term biochemical control remains a goal in addressing acromegaly in the hopes of being able to reverse or stall the progression of disease (). Decreased GH and IGF-1 levels can be accomplished through a number of different methods and mechanisms and have been associated with improved clinical outcomes. Identification of new therapeutic options will supplement the current treatment arsenal to facilitate multifaceted control of abnormalities associated with biochemical dysregulation in acromegaly. While long-term cure remains a current focus with clinicians, increased long-term care of patients will be needed to ensure that comorbidities, QoL, signs and symptoms, and psychosocial functions are properly managed.
Acknowledgments
Financial support for development of this paper was provided by Novartis Pharmaceuticals Corporation. Assistance with medical writing and editing was provided under the direction of the author by Andrea Eckhart and Meredith MacPherson (MedThink SciCom, Inc), with support from Novartis Pharmaceuticals Corporation.
Disclosure
EAC has received research funding from Pfizer. The author reports no other conflicts of interest in this work.
References
- Ben-ShlomoAMelmedSAcromegalyEndocrinol Metab Clin North Am2008371101122viii18226732
- MelmedSAcromegaly pathogenesis and treatmentJ Clin Invest2009119113189320219884662
- KatznelsonLLawsERJrMelmedSEndocrine SocietyAcromegaly: an endocrine society clinical practice guidelineJ Clin Endocrinol Metab201499113933395125356808
- HoldawayIMRajasooryaCEpidemiology of acromegalyPituitary199921294111081170
- BurtonTLe NestourENearyMLudlamWHIncidence and prevalence of acromegaly in a large US health plan databasePituitary Epub2016120
- RosarioPWFrequency of acromegaly in adults with diabetes or glucose intolerance and estimated prevalence in the general populationPituitary201114321722121170595
- ChansonPSalenaveSAcromegalyOrphanet J Rare Dis200831718578866
- AnagnostisPEfstathiadouZAPolyzosSAAcromegaly: presentation, morbidity and treatment outcomes at a single centreInt J Clin Pract201165889690221679284
- BurtonTLe NestourEBancroftTNearyMReal-world comorbidities and treatment patterns of patients with acromegaly in two large US health plan databasesPituitary201316335436223054327
- HoldawayIMBollandMJGambleGDA meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegalyEur J Endocrinol20081592899518524797
- SughrueMEChangEFGabrielRAAghiMKBlevinsLSExcess mortality for patients with residual disease following resection of pituitary adenomasPituitary201114327628321476061
- HoldawayIMRajasooryaRCGambleGDFactors influencing mortality in acromegalyJ Clin Endocrinol Metab200489266767414764779
- CarmichaelJDBonertVSNuñoMLyDMelmedSAcromegaly clinical trial methodology impact on reported biochemical efficacy rates of somatostatin receptor ligand treatments: a meta-analysisJ Clin Endocrinol Metab20149951825183324606084
- KreutzerJVanceMLLopesMBLawsERJrSurgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteriaJ Clin Endocrinol Metab20018694072407711549628
- StarkeRMRaperDMPayneSCVanceMLOldfieldEHJaneJAJrEndoscopic vs microsurgical transsphenoidal surgery for acromegaly: outcomes in a concurrent series of patients using modern criteria for remissionJ Clin Endocrinol Metab20139883190319823737543
- MarquezYTuchmanAZadaGSurgery and radiosurgery for acromegaly: a review of indications, operative techniques, outcomes, and complicationsInt J Endocrinol2012201238640122518121
- CarlsenSMLund-JohansenMSchreinerTPreoperative Octreotide Treatment of Acromegaly study groupPreoperative octreotide treatment in newly diagnosed acromegalic patients with macroadenomas increases cure short-term postoperative rates: a prospective, randomized trialJ Clin Endocrinol Metab20089382984299018492760
- ShenMShouXWangYEffect of presurgical long-acting octreotide treatment in acromegaly patients with invasive pituitary macroadenomas: a prospective randomized studyEndocr J201057121035104421099129
- MaoZGZhuYHTangHLPreoperative lanreotide treatment in acromegalic patients with macroadenomas increases short-term postoperative cure rates: a prospective, randomised trialEur J Endocrinol2010162466166620061334
- MurrayRDKimKRenSGChellyMUmeharaYMelmedSCentral and peripheral actions of somatostatin on the growth hormone-IGF-I axisJ Clin Invest2004114334935615286801
- MarinaDBurmanPKloseMTruncated somatostatin receptor 5 may modulate therapy response to somatostatin analogues-observations in two patients with acromegaly and severe headacheGrowth Horm IGF Res201525526226726188991
- ColaoAAuriemmaRSPivonelloRThe effects of somatostatin analogue therapy on pituitary tumor volume in patients with acromegalyPituitary201619221022126290466
- FredaPUKatznelsonLvan der LelyAJReyesCMZhaoSRabinowitzDLong-acting somatostatin analog therapy of acromegaly: a meta-analysisJ Clin Endocrinol Metab20059084465447315886238
- BevanJSClinical review: the antitumoral effects of somatostatin analog therapy in acromegalyJ Clin Endocrinol Metab20059031856186315613435
- GiustinaAChansonPKleinbergDAcromegaly Consensus GroupExpert consensus document: a consensus on the medical treatment of acromegalyNat Rev Endocrinol201410424324824566817
- LosaMMortiniPUrbazLRibottoPCastrignanóTGiovanelliMPresurgical treatment with somatostatin analogs in patients with acro-megaly: effects on the remission and complication ratesJ Neurosurg2006104689990616776333
- BrunsCLewisIBrinerUMeno-TetangGWeckbeckerGSOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profileEur J Endocrinol2002146570771611980628
- ColaoABronsteinMDFredaPPasireotide C2305 Study GroupPasireotide versus octreotide in acromegaly: a head-to-head superiority studyJ Clin Endocrinol Metab201499379179924423324
- GadelhaMRBronsteinMDBrueTPasireotide C2402 Study GroupPasireotide versus continued treatment with octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trialLancet Diabetes Endocrinol201421187588425260838
- TrainerPJDrakeWMKatznelsonLTreatment of acromegaly with the growth hormone-receptor antagonist pegvisomantN Engl J Med2000342161171117710770982
- Somavert [package insert]New York, NYPfizer2016
- van der LelyAJBillerBMBrueTLong-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDYJ Clin Endocrinol Metab20129751589159722362824
- HodishIBarkanALong-term effects of pegvisomant in patients with acromegalyNat Clin Pract Endocrinol Metab20084632433218431372
- HoflandLJFeeldersRAde HerderWWLambertsSWPituitary tumours: the sst/D2 receptors as molecular targetsMol Cell Endocrinol20103261–2899820438803
- AbsRVerhelstJMaiterDCabergoline in the treatment of acromegaly: a study in 64 patientsJ Clin Endocrinol Metab19988323743789467544
- GadelhaMRKasukiLKorbonitsMNovel pathway for somatostatin analogs in patients with acromegalyTrends Endocrinol Metab201324523824623270713
- Duran-PradoMSaveanuALuqueRMA potential inhibitory role for the new truncated variant of somatostatin receptor 5, sst5TMD4, in pituitary adenomas poorly responsive to somatostatin analogsJ Clin Endocrinol Metab20109552497250220233783
- OriolaJLucasTHalperinIGermline mutations of AIP gene in somatotropinomas resistant to somatostatin analoguesEur J Endocrinol2012168191323038625
- JalladRSTrarbachEBDuarteFHJorgeAABronsteinMDInfluence of growth hormone receptor (GHR) exon 3 and -202A/C IGFBP-3 genetic polymorphisms on clinical and biochemical features and therapeutic outcome of patients with acromegalyPituitary201518566667325552351
- FredaPUMonitoring of acromegaly: what should be performed when GH and IGF-1 levels are discrepant?Clin Endocrinol (Oxf)200971216617019226264
- FeenstraJde HerderWWten HaveSMCombined therapy with somatostatin analogues and weekly pegvisomant in active acromegalyLancet200536594711644164615885297
- HighamCEAtkinsonABAylwinSEffective combination treatment with cabergoline and low-dose pegvisomant in active acromegaly: a prospective clinical trialJ Clin Endocrinol Metab20129741187119322278424
- PetrossiansPBorges-MartinsLEspinozaCGross total resection or debulking of pituitary adenomas improves hormonal control of acromegaly by somatostatin analogsEur J Endocrinol20051521616615762188
- KaravitakiNTurnerHEAdamsCBSurgical debulking of pituitary macroadenomas causing acromegaly improves control by lanreotideClin Endocrinol (Oxf)200868697097518031313
- ColaoAAttanasioRPivonelloRPartial surgical removal of growth hormone-secreting pituitary tumors enhances the response to somatostatin analogs in acromegalyJ Clin Endocrinol Metab2006911859216263832
- EugsterEAPescovitzOHGigantismJ Clin Endocrinol Metab199984124379438410599691
- MercadoMGonzalezBVargasGSuccessful mortality reduction and control of comorbidities in patients with acromegaly followed at a highly specialized multidisciplinary clinicJ Clin Endocrinol Metab201499124438444625210882
- DelaroudisSPEfstathiadouZAKoukoulisGNAmelioration of cardiovascular risk factors with partial biochemical control of acromegalyClin Endocrinol (Oxf)200869227928418194486
- GurnellMPowlsonASCardiovascular disease and sleep disordered breathing in acromegalyNeuroendocrinology20161031758526227953
- KepicogluHHatipogluEBulutIDariciEHizliNKadiogluPImpact of treatment satisfaction on quality of life of patients with acromegalyPituitary201417655756324337714
- MangupliRCamperosPWebbSMBiochemical and quality of life responses to octreotide-LAR in acromegalyPituitary201417649549924178448
- PaisleyANRowlesSVRobertsMETreatment of acromegaly improves quality of life, measured by AcroQolClin Endocrinol (Oxf)200767335836217555502
- MattaMPCoutureECazalsLVezzosiDBennetACaronPImpaired quality of life of patients with acromegaly: control of GH/IGF-I excess improves psychological subscale appearanceEur J Endocrinol2008158330531018299462
- Leon-CarrionJMartin-RodriguezJFMadrazo-AtutxaAEvidence of cognitive and neurophysiological impairment in patients with untreated naive acromegalyJ Clin Endocrinol Metab20109594367437920554710
- TiemensmaJBiermaszNRvan der MastRCIncreased psychopathology and maladaptive personality traits, but normal cognitive functioning, in patients after long-term cure of acromegalyJ Clin Endocrinol Metab20109512E392E40220843947
- CaronPJBevanJSPetersennSPRIMARYS InvestigatorsTumor shrinkage with lanreotide autogel 120 mg as primary therapy in acromegaly: results of a prospective multicenter clinical trialJ Clin Endocrinol Metab20149941282129024423301
- NeggersSJvan AkenMOde HerderWWQuality of life in acromegalic patients during long-term somatostatin analog treatment with and without pegvisomantJ Clin Endocrinol Metab200893103853385918647806
- PlöckingerUMedical therapy of acromegalyInt J Endocrinol2012201226895722550484
- FleseriuMAdvances in the pharmacotherapy of patients with acromegalyDiscov Med2014179632933824979253
- RobertsJLindenMCervinCTibergFOctreotide fluid crystal provides sustained octreotide bioavailability and similar IGF1 suppression to that of octreotide LAR (Sandostatin LAR): randomized, open-label, phase 1, repeat-dose study in healthy volunteers [abstract P914]Endocr Abstr201435329
- TibergFRobertsJCervinCOctreotide s.c. depot provides sustained octreotide bioavailability and similar IGF-1 suppression to octreotide LAR in healthy volunteersBr J Clin Pharmacol201580346047226076191
- MelmedSPopovicVBidlingmaierMSafety and efficacy of oral octreotide in acromegaly: results of a multicenter phase III trialJ Clin Endocrinol Metab201510041699170825664604
- DuarteFHJalladRSBronsteinMDClomiphene citrate for treatment of acromegaly not controlled by conventional therapiesJ Clin Endocrinol Metab201510051863186925738590
