Abstract
Background
There is a paucity of evidence regarding the association between obstructive sleep apnea (OSA) and patients undergoing percutaneous coronary intervention (PCI) for coronary artery disease. We sought to investigate whether OSA affects the clinical outcomes of patients undergoing PCI.
Patients and methods
All enrolled individuals treated with PCI were evaluated for OSA by polysomnography. The primary end point was defined as major adverse cardiac events (MACEs) at 2 years, including cardiac death, myocardial infarction (MI), and/or target vessel revascularization.
Results
A total of 340 consecutive patients undergoing PCI were assigned to the OSA (n=152, apnea–hypopnea index ≥15) and non-OSA (n=188, apnea–hypopnea index <15) groups. The incidence of OSA in patients with coronary artery disease undergoing PCI was 44.7%. Patients in the OSA group had more three-vessel disease (34.9%), increased number of total implanted stents (3.3±2.0), and longer total stent length (83.8±53.1 mm) when compared to the non-OSA group (23.4%, P=0.020; 2.8±1.9, P=0.007; 68.7±48.4, P=0.010). After a median follow-up of 2 years, the incidence of MACEs was significantly higher in patients with OSA (25.0% vs 16.0%, P=0.038), mainly driven by the increased periprocedural MI (19.2% vs 11.2%, P=0.038) in the OSA group. By Cox regression multivariable analysis, the independent predictor of MACEs was OSA (hazard ratio: 1.962, 95% confidence interval: 1.036–3.717, P=0.039).
Conclusion
There was a high prevalence of moderate-to-severe OSA in patients undergoing PCI, and OSA was associated with significantly increased MACE rate, mainly due to the increase in periprocedural MI rate.
Introduction
Obstructive sleep apnea (OSA), which presents in ~9% of females and 24% of males, is characterized by repetitive upper airway obstruction in breathing during sleep and is usually associated with hypertension, arrhythmia, heart failure, insulin resistance, and cerebrovascular accident.Citation1,Citation2 It has also been shown that the prevalence of OSA in patients with coronary artery disease (CAD) was two times more than that in those without CAD, with increased risk of mortality and myocardial infarction (MI), which was believed to be caused by hypoxia, enhanced inflammation, oxidative stress, and endothelial dysfunction.Citation1,Citation3,Citation4 So far, percutaneous coronary intervention (PCI) has been the crucial treatment for symptomatic CAD; however, there is a paucity of evidence regarding the association between OSA and patients undergoing PCI. In 89 consecutive patients with acute coronary syndrome (ACS) treated with PCI, the incidence of cardiac death, reinfarction, and target vessel revascularization (TVR) at 8-month follow-up was 23.5% in the OSA group, which was significantly higher than 5.3% in patients without OSA.Citation5 However, the advanced devices, especially the new generation of drug-eluting stent (DES), were not used in the small-scale study with short follow-up. Therefore, this prospective study is designed to address the long-term clinical impact of OSA in patients undergoing PCI.
Patients and methods
Study population
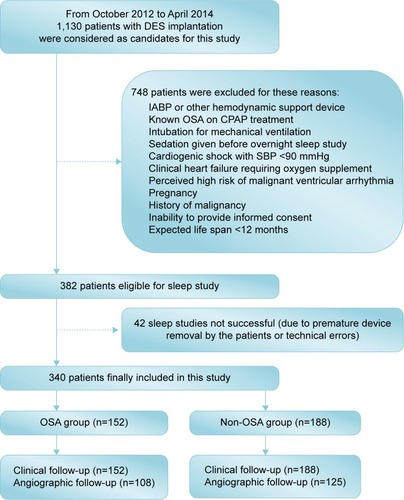
From October 2012 to April 2014, a total of 1,130 consecutive real-world patients who were treated with DES implantation at our center were considered as candidates for this study. Finally, 340 patients were included in the study according to the inclusion and exclusion criteria. Inclusion criteria were as follows: age >18 years and successful PCI in at least one major epicardial coronary artery. Exclusion criteria are shown in . Of note, patients with OSA receiving any relevant treatments were excluded. The protocol was approved by the Institutional Ethics Committee of Nanjing First Hospital, and written informed consent was obtained from all patients.
Percutaneous coronary intervention
All interventional procedures were performed in accordance with the current guidelines. Use of the type of DES, glycoprotein IIb/IIIa inhibitors, intravascular ultrasound, fractional flow reserve, and optical coherence tomography was at the operator’s discretion. A loading dose of clopidogrel (300 mg) or ticagrelor (180 mg) was administered before the index procedure. Total creatine kinase, creatine kinase-myocardial band, and troponin were dynamically measured until 72 hours postprocedure. After the intervention, all patients received 100 mg/d aspirin indefinitely and clopidogrel (75 mg/d) or ticagrelor (90 mg/d bid) for at least 12 months.
Sleep study
All overnight sleep studies for eligible patients were conducted using the Embletta Gold standardized level-3 portable diagnostic system (Natus Medical Inc., Ontario, Canada) 7–10 days after the index PCI procedure,Citation6,Citation7 which included nasal airflow, thoracoabdominal movements, arterial oxygen saturation, snoring episodes, limb movement, electrocardiogram, and body position. All sleep studies were reviewed by an independent sleep specialist according to the guidelines,Citation8 and the poor quality of sleep tracings were excluded from the final analysis of the sleep data. Two independent sleep technologists with registered polysomnographic technologist credentials manually analyzed all studies. The respiratory events of sleep study were performed in accordance with the American Association of Sleep Medicine Guidelines.Citation8 The primary measurement of the sleep study was the apnea–hypopnea index (AHI), which “was used to stratify patients” into the OSA (AHI ≥15) and non-OSA groups (AHI <15).Citation9
Study end points and definitions
The primary end point was the rate of major adverse cardiac events (MACEs) at 2 years, including cardiac death, MI, and/or TVR. The safety end point was the occurrence of stent thrombosis (ST). All deaths were considered cardiac in origin unless a noncardiac cause was confirmed clinically or at autopsy. MI was diagnosed in accordance with the Society for Cardiovascular Angiography and Interventions definition.Citation10 Target lesion revascularization, TVR, and ST were defined according to the Academic Research Consortium definition.Citation11
Follow-up
Clinical follow-up was performed either by telephone or through a clinical office visit at 1 month, 6 months, and 12 months, and every year thereafter. Repeat coronary angiography was scheduled at 12 months after the indexed procedure unless clinical reasons indicated earlier. An independent committee that was blinded to the study assessed all clinical events.
Quantitative coronary angiography
Quantitative coronary angiographic analysis (QCA) at baseline, post-stenting, and at follow-up was performed offline using edge detection techniques (CAAS II, Version 5.0; Pie Medical, Maastricht, the Netherlands) by an independent core laboratory (China Cardiovascular Research Foundation, Beijing, People’s Republic of China). QCA variables included reference vessel diameter, minimal lumen diameter, acute gain, late lumen loss, and net gain, which were defined in our previous study.Citation12
Statistical analysis
The distribution of continuous variables was assessed by the Kolmogorov–Smirnov test. Categorical variables were expressed as frequencies or percentages and compared using chi-square statistics or Fisher’s exact test. Continuous variables were summarized as mean ± SD or median and compared using Student’s t-test (for normal data) and Mann–Whitney U-test (for non-normally distributed variables). Survival curves were generated by the Kaplan–Meier method and compared using the log-rank test. Hazard ratios (HR) are presented along their 95% confidence interval (CI). Univariable and multivariable Cox proportional hazard models were applied to identify potential factors that correlated with MACEs. The variables showing statistical significance or a trend (P<0.1) in univariable Cox model were subjected to multivariable analysis with a forced-entry method. A P-value <0.05 was considered statistically significant, except for P-value <0.01 in univariable analysis to minimize the probability of type I error. All analyses were performed using the statistical program SPSS 16.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline clinical and sleep characteristics
A total of 340 patients (average age: 64.69±10.38 years) with DES implantation were finally enrolled and assigned to the OSA (n=152, 44.7%) and non-OSA (n=188, 55.3%) groups stratified by the AHI. Baseline clinical and sleep characteristics are shown in . None of the 340 study patients had received treatments for OSA. Ten percent of the individuals were admitted with stable angina and the remaining with ACS. Patients in the OSA group were more likely to be males (78.9%) and had greater body mass index (BMI; 25.4±2.9 kg/mm2) and larger left ventricular end diastolic diameter (LVDd; 50.6±6.8 mm), when compared to the non-OSA group (68.6%, P=0.032; 24.5±3.1 kg/mm2, P=0.007; 48.2±4.6 mm, P=0.003). The median AHI of 340 enrolled patients was 13.8, and the median time for SpO2 level <90% was 16.3 minutes in the OSA group, which was significantly higher than the >5.1 minutes in the non-OSA group (P<0.001).
Table 1 Baseline clinical and sleep characteristics of the OSA and non-OSA groups
Lesions and procedural characteristics
shows that patients in the OSA group had more three-vessel disease (34.9%), increased number of total implanted stents (3.3±2.0), and larger total stent length (83.8±53.1 mm) when compared to the non-OSA group (23.4%, P=0.020; 2.8±1.9, P=0.007; 68.7±48.4, P=0.010). As a result, the OSA group had higher procedural time (64.0±49.7 minutes vs 52.8±39.6 minutes, P=0.026).
Table 2 Angiographic and procedural characteristics of the OSA and non-OSA groups
OSA and cardiovascular events
After a median follow-up of 2 years, there were 38 (25.0%) composite MACEs in the OSA group and 30 (16.0%) in the non-OSA group (P=0.038), which were mainly driven by the more periprocedural MI (19.2%) in the OSA group compared to the non-OSA group (11.2%, P=0.038; , ). By Cox regression multivariable analysis, the independent predictor of MACEs was OSA (HR: 1.962, 95% CI: 1.036–3.717, P=0.039; ). There were no significant differences in QCA between the OSA and non-OSA groups ().
Figure 2 MACE-free survival rate at 2 years.
Abbreviations: MACEs, major adverse cardiac events; OSA, obstructive sleep apnea.
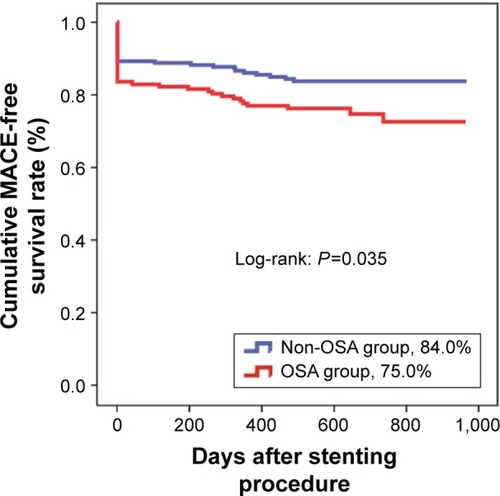
Table 3 Clinical outcomes in the OSA and non-OSA groups
Table 4 Univariate and multivariate analyses for MACEs
Table 5 Quantitative coronary analysis of the OSA and non-OSA groups
Subgroup analysis
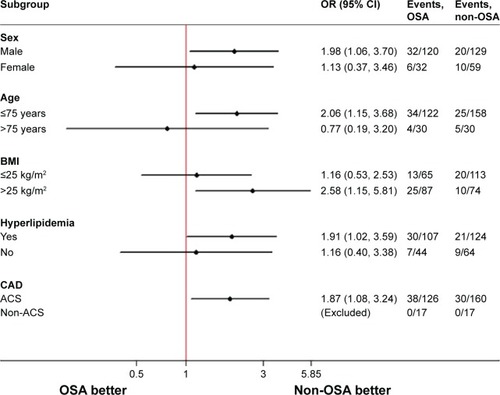
shows the results of subgroup analysis for the associations between OSA and risk of MACEs in patients with DES implantation at 2 years. The incidence of composite MACEs in the OSA group was more remarkable among male patients aged ≤75 years, with BMI >25 kg/m2, hyperlipidemia, and ACS.
Figure 3 Forest plots of 2-year MACE rate in the prespecified subgroups.
Abbreviations: ACS, acute coronary syndrome; BMI, body mass index; CAD, coronary artery disease; CI, confidence interval; MACEs, major adverse cardiac events; OR, odds ratio; OSA, obstructive sleep apnea.
Discussion
The major finding of the current study was that the high prevalence of moderate-to-severe OSA was found in patients with DES implantation, and OSA was associated with significantly increased 2-year composite MACE rate, mainly due to the increment of periprocedural MI rate.
In the current study, we found that 44.7% of patients with CAD undergoing PCI had moderate-to-severe OSA, which was similar to 48.3% and 57% in the current literature.Citation5,Citation13 With chronic intermittent hypoxemia, reoxygenation, and hypercapnia in patients with OSA, there is increased sympathetic activation, induction of oxidative stress, vasoconstriction, and provocation of inflammation leading to endothelial dysfunction and atherosclerosis.Citation1,Citation14,Citation15 Therefore, it is not surprising that the high prevalence of OSA was found in these patients with CAD undergoing PCI. In addition, the current study showed that patients with OSA were more likely to be males and had a significantly greater BMI, which was consistent with published data in the Randomized Intervention With Continuous Positive Airway Pressure in Coronary Artery Disease and Sleep Apnea trial,Citation16 suggesting that age, male, and BMI were predictors of OSA. Moreover, compared to patients without OSA, patients with OSA were more likely to undergo multivessel PCI and had an increased number of DESs implanted during PCI, which might be explained by chronic hypoxia, enhanced inflammation, oxidative stress, and endothelial dysfunction.Citation1,Citation3
The association between OSA and adverse cardiac events has been explored in general population in several studies.Citation3,Citation4,Citation17 The Sleep Heart Health Study enrolling 1,927 men and 2,495 women free of CAD showed that OSA was a significant predictor of incident CAD, MI, revascularization procedure, or cardiovascular death after a median of 8.7-year follow-up.Citation4 In another long-term study including 1,436 patients, OSA increased the risk of coronary events or cardiovascular death, and OSA was an independent risk factor for cardiovascular events, including MI.Citation3 Similar negative effects of OSA could be found in patients with ACS.Citation11,Citation18,Citation19 In 168 consecutive patients with unstable angina or non-ST elevation acute MI, OSA had higher risk of cardiovascular complications during the acute phase of ACS.Citation11 Overall, the current data indicated that OSA has been associated with increased risk of adverse cardiac events in general population or patients with ACS.
Whether OSA adversely affected the prognosis of patients with stent placement for CAD has not been established, and there is a deficiency of evidence regarding the association between OSA and patients undergoing PCI. In 105 patients admitted with ST-segment elevation MI undergoing stent placement, severe OSA carried a negative prognostic impact for these patients after 18-month follow-up.Citation20 Another study which enrolled 89 consecutive patients with ACS treated with PCI showed that the incidence of cardiac death, reinfarction, and TVR at 8-month follow-up was 23.5% in the OSA group, significantly higher than 5.3% in patients without OSA.Citation5 However, the new generation of DES was not used in this small-scale study with short follow-up. Our data confirm this previous evidence and add some novel findings. First, to the best of our knowledge, this is the first study to demonstrate increased periprocedural MI rate of OSA after DES implantation in patients with CAD. Second, a great many angiographic and procedural variables, indicating anatomic severity for coronary disease and the potentially important confounding factors, were included and adjusted in this study. Third, subgroup analysis was conducted to identify a subset of patients undergoing PCI for whom an unfavorable effect of OSA was remarkable. Finally, the differences in techniques, lesion complexity, and DES types used may be impact factors attributed to the discrepancy in several results. However, results from a previous studyCitation5 and ours demonstrated a negative effect of OSA on composite MACE rate in patients undergoing PCI for CAD.
Study limitations
The current study has several limitations. First, none of the 340 study patients had received treatments for OSA, and it was not possible to evaluate the effects of continuous positive airway pressure treatment on cardiovascular outcome. Second, there were 42 sleep studies that were not successful due to premature device removal by the patients or technical errors, and it might not be possible to conduct a repeat sleep study in consideration of the short hospitalization period in patients after PCI. Third, patients were not tested for sleep data again in the follow-up. Thus, there was lack of information regarding post-discharge sleep data and the timing of cardiac events during the follow-up.
Conclusion
The current study showed a high prevalence of moderate-to-severe OSA in patients with DES implantation for CAD, and OSA was associated with significantly increased 2-year composite MACE rate, mainly due to the increment of periprocedural MI rate.
Acknowledgments
The study is supported by the Jiangsu Provincial Special Program of Medical Science (BL2013001).
Disclosure
The authors report no conflicts of interest in this work.
References
- SomersVKWhiteDPAminRSleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular NursingJ Am Coll Cardiol200852868671718702977
- PerkJDe BackerGGohlkeHEuropean Association for Cardiovascular Prevention & Rehabilitation (EACPR)ESC Committee for Practice Guidelines (CPG)European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts)Eur Heart J201233131635170122555213
- ShahNAYaggiHKConcatoJMohseninVObstructive sleep apnea as a risk factor for coronary events or cardiovascular deathSleep Breath201014213113619777281
- GottliebDJYenokyanGNewmanABProspective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health studyCirculation2010122435236020625114
- YuminoDTsurumiYTakagiASuzukiKKasanukiHImpact of obstructive sleep apnea on clinical and angiographic outcomes following percutaneous coronary intervention in patients with acute coronary syndromeAm J Cardiol2007991263017196456
- TiihonenPHukkanenTTuomilehtoHMervaalaETöyräsJEvaluation of a novel ambulatory device for screening of sleep apneaTelemed J E Health200915328328919382867
- Tonelli de OliveiraACMartinezDVasconcelosLFDiagnosis of obstructive sleep apnea syndrome and its outcomes with home portable monitoringChest2009135233033619201709
- BerryRBBudhirajaRGottliebDJRules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep MedicineJ Clin Sleep Med20128559761923066376
- PunjabiNMThe epidemiology of adult obstructive sleep apneaProc Am Thorac Soc20085213614318250205
- MoussaIDKleinLWShahBConsideration of a new definition of clinically relevant myocardial infarction after coronary revascularization: an expert consensus document from the Society for Cardiovascular Angiography and Interventions (SCAI)J Am Coll Cardiol201362171563157024135581
- MauriLHsiehWHMassaroJMHoKKD’AgostinoRCutlipDEStent thrombosis in randomized clinical trials of drug-eluting stentsN Engl J Med2007356101020102917296821
- ChenSLYeFZhangJJDistal left main coronary bifurcation lesions predict worse outcome in patients undergoing percutaneous implantation of drug-eluting stents: results from the Drug-Eluting Stent for the Treatment of Left Main Disease (DISTAL) StudyCardiology2009113426427319246905
- LooGKooCYZhangJImpact of obstructive sleep apnea on cardiovascular outcomes in patients treated with percutaneous coronary intervention: rationale and design of the sleep and stent studyClin Cardiol2014371026126924945037
- CarlsonJTRangemarkCHednerJAAttenuated endothelium- dependent vascular relaxation in patients with sleep apnoeaJ Hypertens19961455775848762200
- Cepeda-ValeryBAcharjeeSRomero-CorralAPressmanGSGamiASObstructive sleep apnea and acute coronary syndromes: etiology, risk, and managementCurr Cardiol Rep2014161053525135347
- GlantzHThunstromEHerlitzJOccurrence and predictors of obstructive sleep apnea in a revascularized coronary artery disease cohortAnn Am Thorac Soc201310435035623952854
- MarinJMCarrizoSJVicenteEAgustiAGLong-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational studyLancet200536594641046105315781100
- SzymanskiFMFilipiakKJHrynkiewicz-SzymanskaAKarpinskiGOpolskiGClinical characteristics of patients with acute coronary syndrome at high clinical suspicion for obstructive sleep apnea syndromeHellenic J Cardiol201354534835424100177
- KonecnyTKuniyoshiFHOrbanMUnder-diagnosis of sleep apnea in patients after acute myocardial infarctionJ Am Coll Cardiol201056974274320723806
- LeeCHKhooSMChanMYSevere obstructive sleep apnea and outcomes following myocardial infarctionJ Clin Sleep Med20117661662122171200