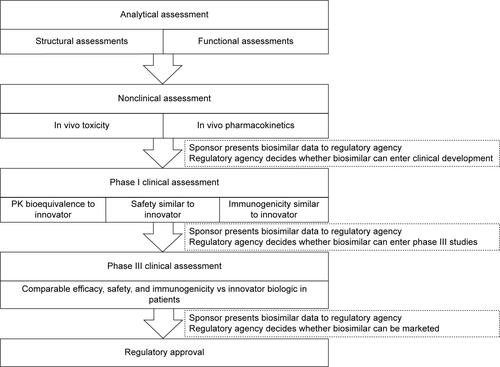
Abstract
Objective
To understand the levels of awareness, usage, and knowledge of biosimilars among patients, caregivers, and the general population in the US and the European Union; perceptions of biosimilars compared to originator biologics; perceived benefits and drawbacks of clinical trials; and whether advocacy groups impact patients’ willingness to try a biosimilar.
Methods
An international survey was conducted which contained up to 56 closed-ended (requiring yes/no or ranking answers) and open-ended questions, depending on the population assigned. The survey was divided into distinct sections, including medication-class awareness, usage, and knowledge about biologic and biosimilar therapies; perceptions of clinical trials; and involvement in advocacy groups. Interviews were conducted in adults categorized as: 1) diagnosed: patients with inflammatory bowel disease including Crohn’s disease and ulcerative colitis, rheumatoid arthritis, psoriasis, breast cancer, lung cancer, colorectal cancer, or non-Hodgkin’s lymphoma; 2) diagnosed advocacy: individuals with these diseases who participated in patient support groups; 3) caregiver: has a loved one with these conditions and is involved in medical decisions; 4) general population: aged 18–64 years, without these conditions. Statistical analyses among groups within a region (US or EU) used column proportions test with a 95% confidence interval.
Results
In all, 3,198 individuals responded. Awareness about biologic therapies was significantly higher in diagnosed, diagnosed advocacy, and caregiver groups (45%–78%) versus general population (27%; P<0.05). Across all groups, awareness of biosimilars was low; only 6% of the general population reported at least a general impression of biosimilars. Awareness was significantly higher in the diagnosed advocacy group (20%–30%; P<0.05). Gaps in knowledge about biosimilars included safety, efficacy, and access to these agents. Respondents had generally positive perceptions of clinical trials, although barriers to participation were identified.
Conclusion
An immediate need exists for patient education about biosimilars and clinical trials to ensure educated and informed decisions are made about biosimilar use.
Supplementary materials
Table S1 Demographics
Acknowledgments
Medical writing support was provided by Christina McManus, PhD, of Engage Scientific Solutions, and funded by Pfizer Inc. The data was accepted as an abstract and poster for presentation at the International Society for Pharmaco-economics and Outcomes Research 18th Annual European Congress (ISPOR-EU), November 7–11, 2015, Milan, Italy. This study was sponsored by Pfizer Inc. The sponsor contributed to the design and conduct of the study, collection and analysis of data, and review of the manuscript. This study was a survey and no interventions were administered; written informed consent and ethics committee (EC)/institutional review board (IRB) approval were not required. In agreement with the journal policy, the authors declare they confirm all patient/personal identifiers have been removed or disguised, so that the patient/person(s) described are not identifiable and cannot be identified through the details of the paper.
Author contributions
All authors were responsible for the interpretation of the data, the final content of the manuscript, and the decision to submit for publication. All authors have made substantial contributions to this manuscript including all of the following: 1) the conception and design of the study, acquisition of data, and/or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content, and 3) final approval of the version to be submitted.
Disclosure
Ira Jacobs, Ena Singh, K Lea Sewell, and Lesley G Shane are current employees of Pfizer Inc. and hold stock or stock options in Pfizer Inc. Ahmad AL-Sabbagh was an employee of Pfizer Inc. at the time of conducting this study and holds stock or stock options in Pfizer Inc. The authors report no other conflicts of interest in this work.